 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(4); 2023 > Article |
|
Abstract
Purpose
Methods
Results
Supplementary material
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Fig. 1.
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig. 1.
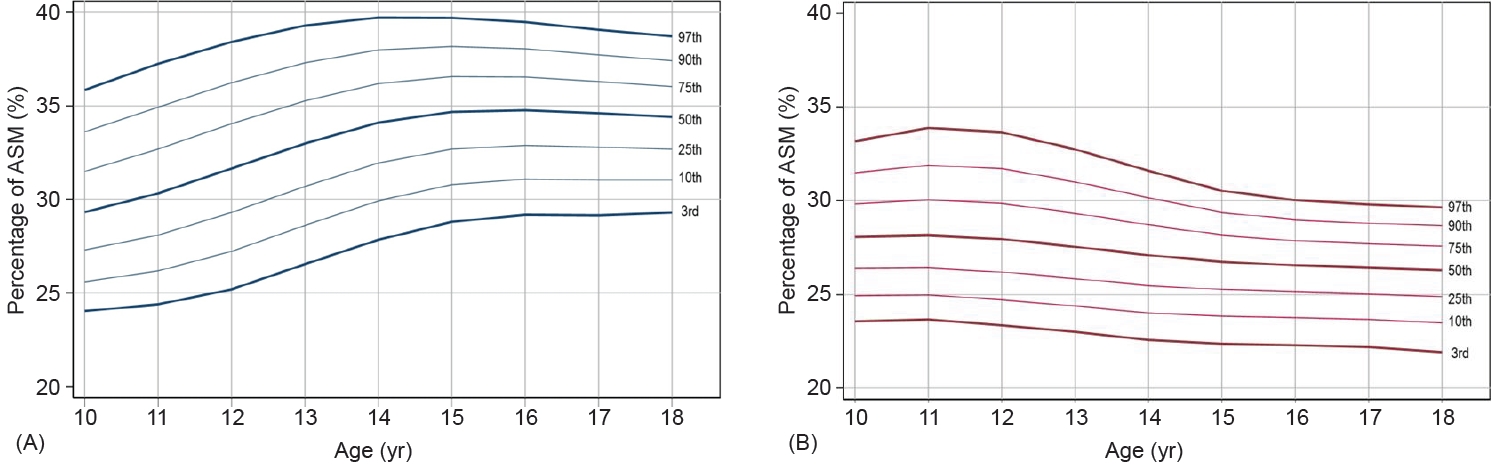
Fig. 2.

Table 1.
Table 2.
| Variable | Total | Boys | Girls | P-value |
|---|---|---|---|---|
| No. (%) | 1,174 | 613 (52.7) | 561 (47.3) | |
| Age (yr) | 13.9±0.1 | 13.9±0.1 | 13.9±0.1 | 0.888 |
| Height (cm) | 161.1±0.4 | 164.8±0.6 | 157.0±0.4 | <0.001 |
| Weight (kg) | 54.3±0.5 | 57.7±0.7 | 50.4±0.6 | <0.001 |
| BMI z-score | -0.04±0.04 | -0.04±0.06 | -0.04±0.06 | 0.959 |
| BMI category | 0.226 | |||
| Normal | 935 (79.6) | 479 (78.1) | 456 (81.3) | |
| Overweight | 127 (10.8) | 67 (10.9) | 60 (10.7) | |
| Obesity | 112 (9.5) | 67 (10.9) | 45 (8.0) | |
| Household income (quintile) | 0.111 | |||
| 1Q, lowest | 160 (13.6) | 79 (12.9) | 81 (14.4) | |
| 2Q | 254 (21.6) | 125 (20.4) | 129 (23.0) | |
| 3Q | 271 (23.1) | 131 (21.4) | 140 (25.0) | |
| 4Q | 237 (20.2) | 132 (21.5) | 105 (18.7) | |
| 5Q, highest | 252 (21.5) | 146 (23.8) | 106 (18.9) | |
| Daily energy intake (kcal) | 2,083.1±30.8 | 2,311.2±41.8 | 1,829.1±39.5 | <0.001 |
| ASM (kg)† | 15.7±0.2 | 18.3±0.2 | 13.2±0.1 | <0.001 |
| PASM (%)† | 29.8±0.2 | 32.8±0.2 | 26.9±0.2 | <0.001 |
| PASM z-score | -0.02±0.05 | 0.09±0.06 | -0.14±0.07 | 0.005 |
| Fat mass (kg) | 14.9±0.2 | 13.7±0.3 | 16.4±0.3 | <0.001 |
| WC (cm) | 67.4±0.3 | 68.3±0.4 | 66.3±0.4 | <0.001 |
| Systolic BP (mmHg) | 107.5±0.4 | 116.1±0.5 | 104.6±0.5 | <0.001 |
| Diastolic BP (mmHg) | 67.1±0.3 | 68.0±0.4 | 66.1±0.4 | <0.001 |
| Triglyceride (mg/dL)† | 76.6±1.7 | 76.3±2.4 | 77.0±1.8 | 0.792 |
| HDL-C (mg/dL)† | 48.1±0.4 | 46.6±0.4 | 49.7±0.5 | <0.001 |
| LDL-C (mg/dL)† | 88.6±0.9 | 84.6±1.2 | 93.2±1.1 | <0.001 |
| FPG (mg/dL)† | 88.7±0.2 | 89.3±0.3 | 88.0±0.3 | 0.001 |
| Insulin (mIU/L)† | 12.4±0.2 | 12.2±0.3 | 12.7±0.3 | 0.169 |
| Metabolic syndrome | 57 (4.9) | 36 (5.9) | 21 (3.7) | 0.090 |
| Insulin resistance | 185 (15.8) | 91 (14.9) | 94 (16.8) | 0.369 |
| PsiMS | 4.17±0.02 | 4.19±0.02 | 4.16±0.02 | 0.181 |
| HOMA-IR† | 2.72±0.04 | 2.68±0.06 | 2.75±0.06 | 0.398 |
| TyG index† | 8.11±0.02 | 8.11±0.03 | 8.11±0.02 | 0.971 |
Values are presented as weighted mean±standard error or as number (%).
BMI, body mass index; Q, quintile; ASM, appendicular skeletal muscle mass; PASM, percentage of appendicular skeletal muscle mass; WC, waist circumference; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose; PsiMS, pediatric simple metabolic syndrome score; HOMA-IR, the homeostasis model assessment of insulin resistance; TyG index, triglyceride glucose index.
Overweight is defined as BMI 85th≤95th percentile for age and sex, obesity is defined as BMI ≥95th percentile for age and sex.
Table 3.
References
- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 2,070 View
- 133 Download
- Related articles in APEM






