 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(1); 2023 > Article |
|
Abstract
Purpose
This study aimed to describe the prevalence of vitamin D deficiency in Indonesian children and adolescents.
Methods
This was a meta-analysis of prevalence using the Hartung-Knapp-Sidik-Jonkman method with a random effects model. A prediction interval was used to estimate true effects. We searched PubMed, MEDLINE, Cochrane Library, Science Direct, Google Scholar, and 3 Indonesian databases (Indonesian Scientific Journal Database, Neliti, and Indonesia One Search). We included cross-sectional or case-control studies that provided data on the prevalence of vitamin D deficiency. We excluded case reports, case series, cohort studies, or studies outside Indonesia. We computed point prevalence by dividing the number of children with hypovitaminosis D by the total number of subjects in that study. This review was registered with PROSPERO (International Prospective Register of Systematic Reviews) (CRD42022329814).
Results
Of 1,397 manuscripts identified, 7 were included in this review. A total of 5,870 children were included in this meta-analysis, ranging in age from 6 months to 19 years. The prevalence of hypovitaminosis D in Indonesia was calculated as 33% (95% confidence interval [CI], 9–56) and was higher in females (60% [95% CI, 58–62]) than in males (40% [95% CI, 38–42]). Mean serum vitamin D level was 22.74 ng/mL (95% CI, 16.95–30.51) with a prediction interval of 15.96 ng/mL to 29.52 ng/mL.
· The prevalence of vitamin D deficiency amongst Indonesian children is lacking. Our meta-analysis found that the prevalence of hypovitaminosis D was 33% and was higher in females (60% vs. 40%). The mean serum vitamin D level was 22.74 ng/mL.
Vitamin D affects the health of skeletal and extraskeletal organs and is a public health concern [1]. Vitamin D deficiency (hypovitaminosis D) has been found to cause rickets and is associated with psoriasis, muscle weakness, cardiomyopathy, increased risk of infections, autoimmune diseases, atopy, cancer, increased risk of cardiac-related deaths, obesity, diabetes, and metabolic syndrome [2-5]. During the pandemic, vitamin D gained attention as deficiency was associated with higher mortality in patients with severe coronavirus disease-19 [6,7].
Several studies have found that vitamin D deficiency is prevalent worldwide. Numerous epidemiological studies and reviews have concluded that vitamin D deficiency is not just a hype but a pandemic in itself [8,9]. In some countries, the government has taken corrective actions to rectify this problem, with successful outcomes in some parts of the world [10,11].
Certain populations are considered at higher risk of vitamin D deficiency than the general population, namely infants, children, pregnant women, and women of childbearing age, due to their increased metabolism and dietary need for vitamin D [12,13]. There is increasing evidence that vitamin D deficiency in childhood and adolescents contributes to noncommunicable diseases in adulthood [14].
Epidemiological data are lacking in Indonesia, with the most recent having been collected a decade ago and likely not reflecting Indonesia's current vitamin D status [15]. Reviews of vitamin D deficiency in Asia have not included Indonesian populations [16,17]. The lack of sound data hinders the government in identifying issues, determining the underlying causes, and designing policies to address identified issues [18].
Our primary goal in this systematic review and meta-analysis was to determine the prevalence of hypovitaminosis D in Indonesian children and adolescents. Our secondary aim was to determine mean vitamin D level and the prevalence of sufficient vitamin D level among Indonesian children and adolescents.
We adhered to the PRISMA (Preferred Reporting Items for Systematic Review) 2020 guidelines [19]. The protocol of this review was registered in the PROSPERO (International Prospective Register of Systematic Reviews) database with the registration number CRD42022329814.
The studied population comprised all children and adolescents (0–19 years old) with available serum 25-hydroxyvitamin D (25(OH)D) measurement. The primary outcome of this study was the point prevalence of hypovitaminosis D in Indonesia. Subgroup analyses were performed based on sex, study quality, method used to measure vitamin D, and risk of bias. The cutoff for vitamin D was based on the criteria set by the Indonesian Pediatric Society [20]: 30–100 ng/mL was considered normal; 21–29 ng/mL insufficient; <20 ng/mL deficient, and <5 ng/mL severely deficient. To ensure the uniformity of the data, we divided mean values in nanomoles per liter (nmol/L) by 2.5 to convert values into nanograms per milliliter (ng/mL). Therefore, we set the cutoff for vitamin D deficiency as <20 ng/mL or <50 nmol/L. However, after searching the literature, we realized that some studies utilized different cutoffs. Hence, according to respective study cutoff, we classified subjects into 2 categories based on vitamin D status: hypovitaminosis (insufficient and deficient) or sufficient. We also performed a post hoc subgroup analysis based on our initial cutoff (>50 ng/mL as a normal vitamin D level). Initially, subgroup analyses of prevalence based on dwelling type, city, and skin type were planned. However, due to the limited data available for these subgroups, these results are reported qualitatively. A secondary outcome of this study was mean serum 25(OH)D level and prevalence of sufficient serum 25(OH)D in Indonesian children. There were no intervention or comparator groups.
Inclusion criteria were cross-sectional studies published in English or an Indonesian language. For case-control studies, we chose the control (normal) groups. We did not include neonates as their serum 25(OH)D levels are primarily influenced by their mothers [21]. We included gray literature such as conference abstracts, theses, and dissertations. Exclusion criteria were case reports, case series, cohort studies, reviews, animal studies, and studies performed outside of Indonesia. Children with comorbidities or those who were acutely ill at the time of the study were excluded. We also excluded studies with fewer than 50 samples [22]. To ensure literature saturation, citations were collected from review studies. We also performed citation and hand searching to ensure that all available studies were included.
The literature search was initiated on 29th April 2022 and ended the same day. We searched 5 academic databases: PubMed, MEDLINE, Cochrane Library, Science Direct, and Google Scholar. Three Indonesian databases were also utilized to increase literature saturation: the Indonesian Scientific Journal Database, Neliti, and Indonesia One Search. Keywords used were related to epidemiology ("epidemiology," "prevalence"), vitamin D ("vitamin D deficiency," "hypovitaminosis D," "vitamin D insufficiency," "ergocalciferol," "25-hydroxyvitamin D," "calcifediol," "cholecalciferol"), age ("adolescent," "pediatric," "child," "infant"), and Indonesia ("Indonesia," "Nanggroe Aceh Darussalam," "Sumatera Utara," "Sumatera Selatan," "Sumatera Barat," "Bengkulu," "Riau," "Kepulauan Riau," "Jambi," "Lampung," "Bangka Belitung," "Kalimantan Barat," "Kalimantan Timur," "Kalimantan Selatan," "Kalimantan Tengah," "Kalimantan Utara," "Banten," "DKI Jakarta," "Jawa Barat," "Jawa Tengah," "DI Yogyakarta," "Jawa timur," "Bali," "Nusa Tenggara Timur," "Nusa Tenggara Barat," "Gorontalo," "Sulawesi Barat," "Sulawesi Tengah," "Sulawesi Utara," "Sulawesi Tenggara," "Sulawesi Selatan," "Maluku Utara," "Maluku," "Papua Barat," "Papua"). Medical Subject Heading (MeSH) terms for each database are presented in Supplementary Table 1. All records were entered into Rayyan software, which automatically recognizes duplicates [23]. This software also allows authors to collaborate in selecting relevant studies. Two independent authors (AS and MM) conducted the initial search and imported all findings into Rayyan. Another author (GSO) cross-checked the initial searches. These 3 authors independently screened all available studies. Conflicts were resolved by discussion with an expert (AH). In studies with overlapping time points, we chose the data that provided the largest amount of available information.
Data extraction was carried out independently by 1 author (GSO) and then reviewed by 2 authors (AS and MM) to ensure accuracy. We extracted relevant information of study identification (author and year of publication), study characteristics (location, study design, age of participants, and study period), and vitamin D-related information (vitamin D estimation method, mean level of vitamin D, vitamin D cutoff, and total participants).
The Newcastle-Ottawa scale (NOS) for cross-sectional studies was implemented to assess the quality of the studies. A score of 7–9 on the NOS implies a good quality study, 4–6 indicates a moderate or fair quality study, and a score of 0–3 indicates a poor quality study [24]. To assess the risk of bias, we used Joanna Briggs' Institute criteria for prevalence studies. Studies with a score of 0–3 were considered to have low risk of bias, those with a score of 4–6 were considered to have moderate risk of bias, and those with a score of 7–9 were considered to have a high risk of bias [25]. Three reviewers (GSO, AS, MM) independently assessed the scales, and any discrepancies were discussed with the expert (AH) until a consensus was attained. Missing or insufficient data were requested from the appropriate corresponding author, but none was obtained.
We computed point prevalence by dividing the number of children with hypovitaminosis D by the total number of subjects in that study [26]. We used prediction intervals to assess heterogeneity [27], and between-study heterogeneity was explored with a Galbraith plot [28]. Publication bias was assessed with funnel plot analysis for more than 10 studies [29], Begg and Mazumdar's test was used for rank correlation [30], and Egger test was used to calculate the regression intercept [31]. Trim-and-fill analysis was planned for identification of asymmetry in the funnel plot [32]. Analyses were executed in Stata 17.0 (StataCorp., College Station, TX, USA) using the metaprop command [33], and results were displayed using forest plots.
We identified 1,397 manuscripts, 114 of which were dupli cates. A total of 1,206 records was eliminated after assessment of the titles and abstract, and 77 articles were subjected to a full assessment. A total of 6 articles was included in the final analysis. Fourteen additional articles were collected through citation and hand searching, one of which was included in our meta-analysis [15]. Notable exclusions included one study that did not specify the vitamin D cutoff [34], one study that included Dutch residents of Indonesian ethnicity [35], 2 studies [36,37] generated from the same dataset [38], one poster presentation [39] that was published as a full paper [40], and 5 studies [41-45] that were part of the Southeast Asian Nutrition Surveys (SEANUTS) study [15]. Therefore, 7 studies [15,38,40,46-49] were subjected to systematic re vie w and meta-analysis (Supplementary Fig. 1). The characteristics of the studies and Newcastle-Ottawa scale scores are presented in Table 1, while the risk of bias of each study is presented in Supplementary Table 2.
A total of 5,870 children was included in this meta-analysis, ranging in age from 6 months to 19 years. The overall study period covered 2011–2020, during which the prevalence of hypovitaminosis D in Indonesia was 33% (95% confidence interval [CI], 9–56; I2 =99.76%, P<0.01) (Fig. 1). The Galbraith plot indicated no heterogeneity (Supplementary Fig. 2A). Begg and Mazumdar test for rank correlation gave a P-value of <0.001, indicating possible publication bias. Egger test for a regression intercept yielded a P-value of 0.0027, also indicating possible publication bias.
Subgroup analysis was performed for study quality, the method used to measure vitamin D, and risk of bias. Prevalence rate was higher among studies with a low risk of bias (37%; 95% CI, 9–64) compared to those with a moderate risk of bias (9%; 95% CI, 6–13) (Fig. 2). Five studies had a low risk of bias, while one study had moderate bias. Good quality studies reported higher prevalence rates of hypovitaminosis D (34%; 95% CI, 4–6) than moderate quality studies (13%; 95% CI, 10–16) (Fig. 3). Last, studies that used liquid chromatography-tandem mass spectroscopy (LC-MS) reported a higher prevalence rate (69%; 95% CI, 67–72) than studies that used a non-LC-MS method (26%; 95% CI, 2–49) to measure serum vitamin D (Fig. 4). The prevalence of hypovitaminosis D in studies that used 50 ng/mL as their cutoff was 49% (95% CI, 20–79), compared to 10% (95% CI, 2–18) in studies that used 30 ng/mL as their cutoff (Fig. 5).
Three studies provided sufficient data to analyze the prevalence of hypovitaminosis D by sex. The prevalence rate for hypovitaminosis D in females (60%; 95% CI, 58–62; I2=0%, P=0.48) (Fig. 6) was higher than in males (40%; 95% CI, 38–42; I2=0%, P=0.48) (Fig. 7). The Galbraith plot indicated no heterogeneity in either sex (Supplementary Figs. 2B, C). For the female subgroup, Begg and Mazumdar test for rank correlation gave a P-value of 0.29, indicating no publication bias, consistent with the P-value of 0.61 for the regression intercept obtained from Egger test. Similarly, neither Begg and Mazumdar test for rank correlation (P=0.29) nor Egger test for a regression intercept (P=0.51) indicated the presence of publication bias.
Six studies provided sufficient data to calculate mean vitamin D level among Indonesian children and adolescents. Collectively, the mean serum vitamin D level was 22.74 ng/ml (95% CI, 16.95–30.51 ng/mL), and the prediction interval was 15.96 ng/mL to 29.52 ng/mL. The Galbraith plot indicated no heterogeneity (Supplementary Fig. 3). Begg and Mazumdar test for rank correlation provided a P-value of 0.0085, indicating possible publication bias. Egger test returned a regression intercept with a P-value of 0.0003, indicating possible publication bias. When further categorized by LC-MS use, studies that used LC-MS reported lower mean vitamin D level (21.32; 95% CI, 7.56–60.15) than studies that used non-LC-MS methods (22.80; 95% CI, 18.33–28.34).
Only 37% of children in Indonesia had a sufficient vitamin D level (95% CI, 17–57) (Fig. 8). The Galbraith plot indicated no heterogeneity (Supplementary Fig. 4A). Begg and Mazumdar test for rank correlation gave a P-value of 0.0085, indicating possible publication bias. Egger test for a regression intercept resulted in a P-value of <0.0001, also indicating possible publication bias. The prevalence of sufficient vitamin D levels was higher in males at 54% (95% CI, 44–63; I2=83.76%; P<0.01) (Fig. 9) than females at 46% (95% CI, 37–56; I2=83.76%; P<0.01) (Fig. 10). The Galbraith plot for males indicated no heterogeneity (Supplementary Fig. 4B). Begg and Mazumdar test for rank correlation yielded a P-value of 0.296, indicating no evidence of publication bias. Egger test for a regression intercept yielded a P-value of 0.035, indicating possible publication bias. The Galbraith plot for females indicated no heterogeneity (Supplementary Fig. 4C). Begg and Mazumdar test for rank correlation gave a P-value of 0.296, indicating no evidence of publication bias, while Egger test for a regression intercept yielded a P-value of 0.028, indicating possible publication bias.
Roth et al. [3] stated that a high prevalence of vitamin D deficiency was incidence in >20% of the population of a country. Based on this threshold, Indonesian children and adolescents have a high prevalence rate of hypovitaminosis D at 33%. According to the National Statistics Bureau of Indonesia, there were 88,385 children and adolescents aged 0–19 years in 2021 [50]. Based on the calculated numbers, 29,167 Indonesian children are currently suffering from hypovitaminosis D. This rate is higher than that of adolescent girls in India (25.7%) [51], African children (10.55%) [52], and European children (4%–7%) [9] but lower than rates reported for children in Afghanistan (96.2%) and Pakistan (94%) [16]. The most recent systematic review of the prevalence of vitamin D deficiency among 5 Southeast Asian countries (Thailand, Indonesia, Vietnam, Malaysia, and Cambodia) reported a prevalence range from 0.9% to 96.4% [53]. However, almost all studies conducted to date have implemented different vitamin D cutoffs to determine the prevalence of hypovitaminosis D, complicating direct comparison. The CI for hypovitaminosis D prevalence in this study was 9%, which indicates a true prevalence rate less than 20%.
When compared by sex, females (60%) had a higher prevalence rate of hypovitaminosis D than males (40%). This indicates that 18,146 of the 45,366 male children and adolescents in Indonesia currently suffer from hypovitaminosis D, along with 25,811 of 43,018 young females [50]. Other studies have confirmed this phenomenon [16,54], with one study of an Islamic public school reporting that females had a higher prevalence of vitamin D deficiency than males [40]. One possible explanation for this is that Southeast Asian females wear traditional heavy clothing such as hijabs, which protect the skin from ultraviolet (UV) light [55]. Furthermore, Asian females are concerned about sun exposure and will intentionally avoid or protect themselves from it [17,56]. Other factors that likely contribute to the high prevalence of hypovitaminosis D in Indonesian children and adolescents are malnutrition and darker skin type [38,40,46].
A narrative review was conducted for all factors that could not be synthesized quantitatively. Only 2 studies assessed the effect of Fitzpatrick skin type on vitamin D deficiency [38,40]. Pulungan et al. [40] found that students with Fitzpatrick skin type IV were more likely to have sufficient vitamin D (83.2%) level than nonsufficient vitamin D level (68.1%), although this association was not significant. They also found that more prolonged sun exposure was significantly associated with a sufficient vitamin D level. Wearing long sleeves or skirts did not significantly affect vitamin D sufficiency. However, Oktaria et al. [38] found that infants with Fitzpatrick skin type III and IV had a significantly lower risk of hypovitaminosis D compared to infants with Fitzpatrick skin type II. They also found that prolonged weak sun exposure was an independent and significant risk factor for low vitamin D level in infants.
The prevalence rate of hypovitaminosis D found in this meta-analysis was higher in studies categorized with a low risk of bias and of good quality. While this finding contradicts the importance of selecting only high-quality and appropriate studies for quantitative assessment [57], our metanalysis included only one study with a moderate risk of bias. Our rationalization for subgrouping the prevalence of vitamin D deficiency based on the method used for measurement is that LC-MS has been touted as the "reference standard" [58]. In nearly the same number of studies in each arm (LC-MS vs. non-LC-MS), the prevalence of hypovitaminosis D in the LC-MS arm was almost 3 times as high as that in the non-LC-MS group. This finding signifies the importance of appropriate measurement tools in studies that heavily rely on measurement accuracy, reliability, and validity.
According to the Indonesian Pediatric Society vitamin D cutoff value, Indonesian children and adolescents have insufficient serum vitamin D levels [20]. When assessed according to the predetermined cutoff of 50 ng/mL, the prevalence of hypovitaminosis D was higher than that reported in studies that used a different cutoff. However, this subgroup analysis was performed post hoc, and only 2 studies adhered to this cutoff. Therefore, any interpretations should be considered observational, at best [59]. Mean vitamin D level was higher than the pooled serum vitamin D level reported for South Asian children and adolescents (19.15 ng/mL) [16] but lower than that reported for African children (28.88 ng/mL) [52]. Numerous factors affect serum vitamin D level besides those mentioned previously, such as dietary and vitamin D supplement intake [10], body mass index, physical activity [60], genetic influences [61,62], and infection status [63]. Studies that have used LC-MS have reported a lower mean of serum vitamin D than studies that used non-LC-MS methods. The Vitamin D Standardization Program found widely different prevalence rates of hypovitaminosis D when comparing studies that used unstandardized LC-MS [9].
Less than half of Indonesian children and adolescents have adequate serum vitamin D level. The prevalence rate was likely worse for female children and adolescents than males for the reasons outlined earlier. The high prevalence rate of hypovitaminosis D and low prevalence rate of vitamin D sufficiency pose a problem for women, especially pregnant women, as low serum vitamin D can affect neonates' anthropometrical and neurodevelopmental characteristics [21,64,65].
Our meta-analysis suffers from several limitations. The lack of uniform vitamin D cutoffs has been mentioned as a limitation in numerous previous studies [2,66,67]. We resorted to roughly separating individuals based on self-tested vitamin D level into "normal" and "subnormal" subgroups as many studies did not provide sufficient information to classify the study subjects more robustly. The difficulty in classifying vitamin D levels may explain the wide CI in the prevalence of hypovitaminosis D. However, we feel this rough classification is justified as different guidelines have proposed different cutoffs with particular concerns in mind such as optimal values for skeletal health compared to extraskeletal benefits. We also were unable to perform subgroup meta-analyses based on weather, region, nutritional status, skin type, activities, UV index, dietary and supplement intake, or air pollution index due to differences in how and what data were reported among studies. Some of the included studies did not specify the exact location of their research [15,48], but only one study was conducted outside of Java [49]. Another concern is publication bias. Although the Galbraith plot indicated no publication bias for our meta-analysis, Begg and Egger tests indicated publication bias for all findings except prevalence of hypovitaminosis D in male and female subgroups. However, we searched the literature extensively, including gray abstracts. Therefore, it is possible that unpublished studies exist or that studies that included fewer than 50 participants contributed to this publication bias. We did not utilize the traditional I2 proportion to measure heterogeneity because it does not disclose genuine effect size dispersion [68]. Furthermore, because Cochran Q test is only utilized in conjunction with the DerSimonian-Laird technique, we did not implement this test [69]. Our method of reporting heterogeneity by presenting the prediction interval, however, conveys the true prevalence rate [27].
Strengths of our meta-analysis include that this is the first meta-analysis to measure the hypovitaminosis D prevalence rate in Indonesian children and adolescents. We agree with Cashman that a proper estimate is best derived from nationally representative population-based surveys [70]. The study by Sandjaja et al. [15], which was part of the SEANUTS study, fits this description. However, this study is over a decade old and only sampled 0.009% of the total population. It also did not include children younger than 2 years or older than 12 years, making our meta-analysis more comprehensive with regard to age range. Although our meta-analysis has shortcomings, the information can be used to guide Indonesian government, policymakers, healthcare professionals, and other stakeholders to plan strategies to address the high prevalence of hypovitaminosis D in Indonesia children.
In conclusion, with a prevalence rate of 33%, hypovitaminosis D is a public he alth issue in Indonesi an children and adolescents. More females (60%) than males (40%) suffer from hypovitaminosis D and both these groups are at high risk due to the impact of hypovitaminosis D on growth. Lack of treatment of hypovitaminosis D in childhood may result in potentially lethal and disabling chronic diseases in adulthood that significantly burden individuals and the nation.
To ensure uniformity among studies, there needs to be a better guiding consensus to determine the appropriate vitamin D cutoff level. We suggest using the Indonesian Pediatric Society's cutoff as it is based on local consensus. Publicly accessed, deidentified data need to be made available as well to ensure better data transparency and accessibility. A large population-based study that takes into consideration the phenotype and genotype, latitude and sun exposure, dietary intake and habits, and other relevant factors of Indonesian children and adolescents is urgently needed.
Interventions are required to address the high prevalence of hypovitaminosis D. While supplementation may solve this issue temporarily, it may not be the best approach in Indonesia until further confirmatory studies are done. Dose adjustment of vitamin D supplementation and lifestyle modifications may be important for correcting the significant prevalence of hypovitaminosis D in Indonesia.
Supplementary Materials
Supplementary Tables 1-2 and Figs. 1-4 can be found via https://doi.org/10.6065/apem.2244170.085.
Supplementary Table 1.
Medical subject heading (MeSH) terms and keywords used in each database
Supplementary Table 2.
Assessment of risk of bias using Joanna Briggs Institute (JBI) Checklist
Supplementary Fig. 1.
PRISMA flowchart for selection of included studies
Supplementary Fig. 2.
Galbraith plot of the prevalence of overall hypovitaminosis (A), in the male population (B), and female population (C). CI. confidence interval.
Supplementary Fig. 3.
Galbraith plot of the mean vitamin D level. CI. confidence interval.
Supplementary Fig. 4.
Galbraith plot for the prevalence of overall sufficient vitamin D level (A), in the male population (B), and female population (C). CI. confidence interval.
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig. 1.
Prevalence of pediatric vitamin D deficiency in Indonesia. ES, effect size; CI, confidence interval.

Fig. 2.
Prevalence of pediatric vitamin D deficiency in Indonesia according to risk of bias. ES, effect size; CI, confidence interval.
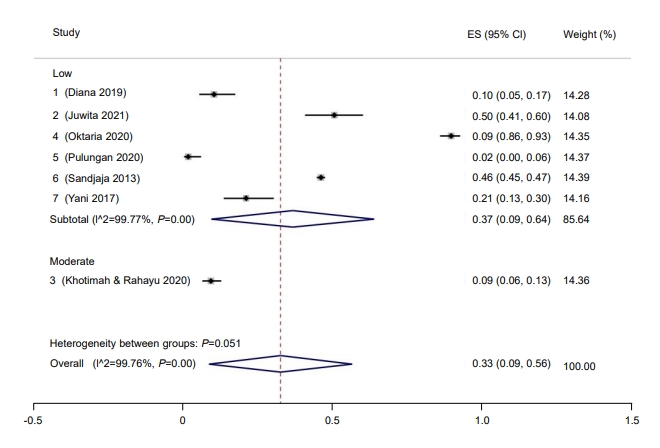
Fig. 3.
Prevalence of pediatric vitamin D deficiency in Indonesia according to study quality. ES, effect size; CI, confidence interval.
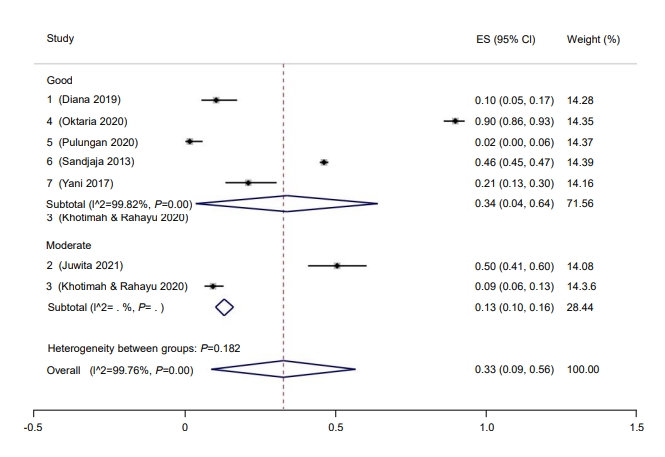
Fig. 4.
Prevalence of pediatric vitamin D deficiency in Indonesia according to machines used. ES, effect size; CI, confidence interval.

Fig. 5.
Prevalence of pediatric vitamin D deficiency in Indonesia according to cutoff. ES, effect size; CI, confidence interval.
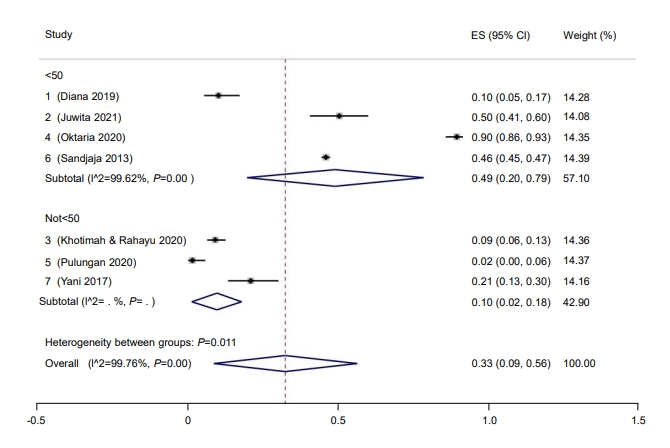
Fig. 6.
Prevalence of male pediatric vitamin D deficiency in Indonesia. ES, effect size; CI, confidence interval.

Fig. 7.
Prevalence of female pediatric vitamin D deficiency in Indonesia. ES, effect size; CI, confidence interval.
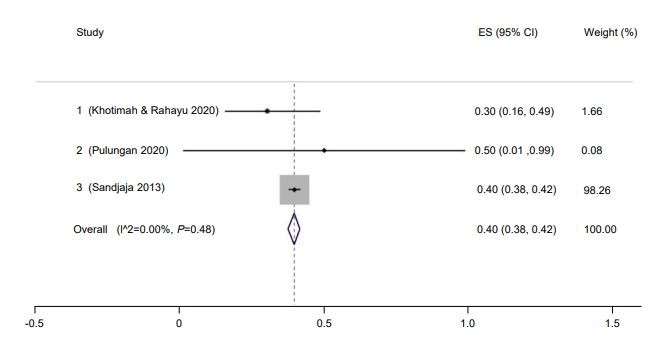
Fig. 8.
Prevalence of children and adolescent with sufficient vitamin D in Indonesia. ES, effect size; CI, confidence interval.
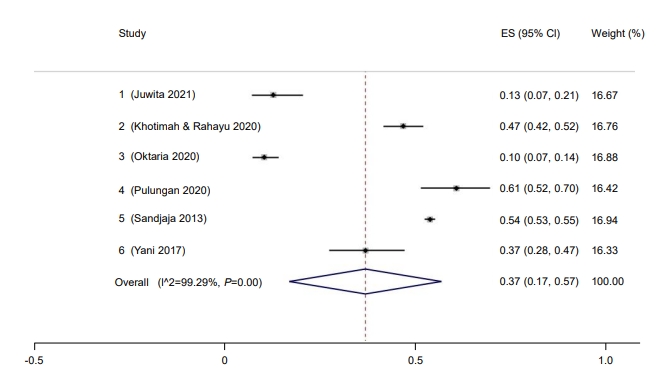
Fig. 9.
Prevalence of male children and adolescent with sufficient vitamin D in Indonesia. ES, effect size; CI, confidence interval.

Fig. 10.
Prevalence of female children and adolescent with sufficient vitamin D in Indonesia. ES, effect size; CI, confidence interval.
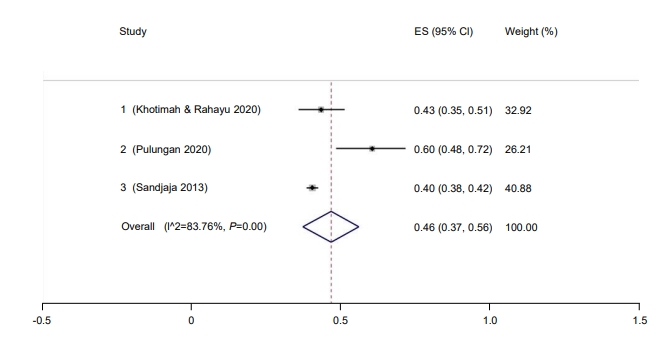
Table 1.
Characteristics of the included studies
| Study | Study period | Study design | Age of participants | Location | Mean vitamin D level (ng/mL) | Vitamin D estimation method | Vitamin D cutoff | Total participants with measured vitamin D |
Newcastle-Ottawa scale |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Total | Classification | |||||||||
| Diana et al. (2019) [46] | August 2013–August 2014 | Repeated cross-sectional study | 6 Months | 30 Villages in 3 subdistricts of Sumedang district, West Java | 89.7 | LC-MS | <50 nmol/L was considered to indicate a low vitamin D level | 116 | 4 | 2 | 3 | 9 | Good |
| Juwita et al. (2021) [47] | 2016–2019 | Cross-sectional study | 23–29 Months old | Sukabumi and Waled, West Java, Indonesia | 50.7 | ELISA | <20 ng/mL=deficiency; 21–29 ng/mL=insufficiency; ≥30 ng/mL=sufficient | 109 | 2 | 2 | 2 | 6 | Moderate |
| Khotimah and Rahayu (2020) [48] | January 2017–May 2020 | Cross-sectional study | 10–19 Years old | Jakarta | N/A | ELISA | 0–11.9 nmol/L=deficiency; 12–20.9 nmol/L=insufficiency; 21–100 nmol/L=sufficiency | 356 | 2 | 1 | 2 | 5 | Moderate |
| Oktaria et al. (2020) [38] | December 2015–December 2017 | Repeated cross-sectional study | 6 Months | 9 PHC and 5 private clinics in Yogya-karta and Kulon Progo | 30 | LC-MS | <50 nmol/L was considered to indicate a low vitamin D level | 249 | 4 | 2 | 3 | 9 | Good |
| Pulungan et al. (2021) [40] | 2012 | Cross-sectional study | 7–12 years old | A public primary school and an Islamic private primary school in Jakarta | 21.85 | RIA | ≤12 ng/mL=deficient; 12–20 ng/mL=insufficient; ≥20 ng/mL=sufficient | 120 | 2 | 2 | 3 | 7 | Good |
| Sandjaja et al. (2013) [15] | 2011 | Cross-sectional study | 2–12 years old | 48 Cities/provinces | 52.34 | Not stated | <50 nmol/L indicated low vitamin D | 4820 | 4 | 2 | 3 | 9 | Good |
| Yani et al. (2017) [49] | March 2014–December 2015 | Case-control | Children younger than 5 years | 22 PHCs in Padang, West Sumatra | 27.57 | ELISA | >30 ng/mL=sufficient, 20–29 ng/mL=insufficient, and 0–19 ng/mL =deficient | 100 | 3 | 1 | 3 | 7 | Good |
References
1. Darnton-Hill I. Public health aspects in the prevention and control of Vitamin deficiencies. Curr Dev Nutr 2019;3:nzz075.



2. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40:1109–51.



3. Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci 2018;1430:44–79.




4. Scragg R, Sluyter JD. Is there proof of extraskeletal benefits from vitamin D supplementation from recent mega trials of vitamin D? JBMR Plus 2021;5:e10459.




5. Siadat ZD, Kiani K, Sadeghi M, Shariat AS, Farajzadegan Z, Kheirmand M. Association of vitamin D deficiency and coronary artery disease with cardiovascular risk factors. J Res Med Sci 2012;17:1052–5.


6. Tomaszewska A, Rustecka A, Lipińska-Opałka A, Piprek RP, Kloc M, Kalicki B, et al. The role of vitamin D in COVID-19 and the impact of pandemic restrictions on vitamin D blood content. Front Pharmacol 2022;13:836738.



7. Dror AA, Morozov N, Daoud A, Namir Y, Yakir O, Shachar Y, et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS One 2022;17:e0263069.



8. Cashman KD. Vitamin D deficiency: a public health issue in high- and low-income countries or just hype? World Rev Nutr Diet 2017;118:206–14.


9. Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 2016;103:1033–44.



10. Cashman KD. Global differences in vitamin D status and dietary intake: a review of the data. Endocr Connect 2022;11:e210282.

13. Mithal A, Kalra S. Vitamin D supplementation in pregnancy. Indian J Endocrinol Metab 2014;18:593–6.



14. Haimi M, Kremer R. Vitamin D deficiency/insufficiency from childhood to adulthood: Insights from a sunny country. World J Clin Pediatr 2017;6:1–9.



15. Sandjaja S, Budiman B, Harahap H, Ernawati F, Soekatri M, Widodo Y, et al. Food consumption and nutritional and biochemical status of 0·5-12-year-old Indonesian children: the SEANUTS study. Br J Nutr 2013;110 Suppl 3:S11–20.


16. Siddiqee MH, Bhattacharjee B, Siddiqi UR, Rahman MM. High burden of hypovitaminosis D among the children and adolescents in South Asia: a systematic review and meta-analysis. J Health Popul Nutr 2022;41:10.




17. Cashman KD, Sheehy T, O'Neill CM. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur J Nutr 2019;58:433–53.



18. Martin EG, Begany GM. Opening government health data to the public: benefits, challenges, and lessons learned from early innovators. J Am Med Inform Assoc 2017;24:345–51.




19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



20. Yati NP, Batubara JRL, Suryawan IWB. Vitamin D: Panduan praktis klinis Ikatan Dokter Anak Indonesia. Jakarta: Badan Penerbit Ikatan Dokter Anak Indonesia. 2018.
21. Judistiani RTD, Madjid TH, Irianti S, Natalia YA, Indrati AR, Ghozali M, et al. Association of first trimester maternal vitamin D, ferritin and hemoglobin level with third trimester fetal biometry: result from cohort study on vitamin D status and its impact during pregnancy and childhood in Indonesia. BMC Pregnancy Childbirth 2019;19:112.




22. Britto C, Pollard AJ, Voysey M, Blohmke CJ. An appraisal of the clinical features of pediatric enteric fever: systematic review and meta-analysis of the age-stratified disease occurrence. Clin Infect Dis 2017;64:1604–11.



23. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210.




24. Wells G, Shea B, O'Connell D, Peterson J, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON), Ottawa Hospital Research Institute. 2014;[cited 2022 Jun 20]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
25. The Joanna Briggs Institute. Checklist for prevalence studies [Internet]. Adelaide (Australia), The Joanna Briggs Institute. 2017;[cited 2022 Jun 20]. Available from: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf.
26. Noordzij M, Dekker FW, Zoccali C, Jager KJ. Measures of disease frequency: prevalence and incidence. Nephron Clin Pract 2010;115:c17–20.



27. Borenstein M. Research Note: In a meta-analysis, the I2 index does not tell us how much the effect size varies across studies. J Physiother 2020;66:135–9.

28. Gao J, Yang L, Zhao J, Wang L, Zou J, Wang C, et al. Comparison of problem-based learning and traditional teaching methods in medical psychology education in China: a systematic review and meta-analysis. PLoS One 2020;15:e0243897.



29. Page M, Higgins J, Sterne J. Chapter 13: Assessing risk of bias due to missing results in a synthesis. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJet al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons. 2019;pp 349–74.
30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.


31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34.



32. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63.


33. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39.




34. Juliaty A, Daud D, Lisal JS. Correlation between vitamin D deficiency and fasting blood glucose levels in obese children. Clin Nutr ESPEN 2021;44:200–3.


35. Tromp IIM, Franco OH, van den Hooven EH, Heijboer AC, Jaddoe VWV, Duijts L, et al. 25-Hydroxyvitamin D concentrations, asthma and eczema in childhood: The generation R study. Clin Nutr 2018;37:169–76.


36. Oktaria V, Danchin M, Triasih R, Soenarto Y, Bines JE, Ponsonby AL, et al. The incidence of acute respiratory infection in Indonesian infants and association with vitamin D deficiency. PLoS One 2021;16:e0248722.



37. Oktaria V, Triasih R, Graham SM, Bines JE, Soenarto Y, Clarke MW, et al. Vitamin D deficiency and severity of pne umoni a in Indonesi an chi ldren. PLoS One 2021;16:e0254488.



38. Oktaria V, Graham SM, Triasih R, Soenarto Y, Bines JE, Ponsonby AL, et al. The prevalence and determinants of vitamin D deficiency in Indonesian infants at birth and six months of age. PLoS One 2020;15:e0239603.



39. Soesanti F, Pulungan A, Tridjaja B, Batubara JRL. Vitamin D profile in healthy children aged 7-12 years old in Indonesia. Int J Pediatr Endocrinol 2013;2013(Suppl 1):P167.



40. Pulungan A, Soesanti F, Tridjaja B, Batubara J. Vitamin D insufficiency and its contributing factors in primary school-aged children in Indonesia, a sun-rich country. Ann Pediatr Endocrinol Metab 2021;26:92–8.




41. Valentina V, Palupi NS, Andarwulan N. Asupan kalsium dan vitamin D pada anak indonesia usia 2-12 tahun. J Teknol Ind Pangan 2014;25:83–9.

42. Ernawati F, Budiman B. Status vitamin D terkini anak Indonesia usia 2,0-12,9 Tahun. Gizi Indonesia 2015;38:73–80.


43. Ernawati F, Syauqy A, Arifin AY, Soekatri MYE, Sandjaja S. Micronutrient deficiencies and stunting were associated with socioeconomic status in indonesian children aged 6-59 months. Nutrients 2021;13:1802.



44. Nguyen Bao KL, Sandjaja S, Poh BK, Rojroongwasinkul N, Huu CN, Sumedi E, et al. The consumption of dairy and its association with nutritional status in the South East Asian Nutrition Surveys (SEANUTS). Nutrients 2018;10:759.



45. Poh BK, Rojroongwasinkul N, Nguyen BK, Ruzita AT, Yamborisut U, et al. 25-hydroxy-vitamin D demography and the risk of vitamin D insufficiency in the South East Asian Nutrition Surveys (SEANUTS). Asia Pac J Clin Nutr 2016;25:538–48.

46. Diana A, Purnamasari DM, Rahmannia S, Luftimas DE, Haszard JJ, Gibson RS, et al. Multimicronutrient biomarkers are related to anemia during infancy in indonesia: a repeated cross-sectional study. Curr Dev Nutr 2019;3:nzz022.



47. Juwita F, Gumilang L, Risan NA, Dhamayanti M. The association of vitamin D and neurodevelopmental status among 2 years old infants. Glob Pediatr Health 2021;8:2333794. X211034075.




48. Khotimah E, Rahayu L. Analisa kadar vitamin D 25 (OH) total pada pasien usia 10–19 tahun yang melakukan pemeriksaan di prodia kelapa Gading-Jakarta. Jurnal Bidang Ilmu Kesehatan 2020;10:160–8.


49. Yani FF, Lipoeto NI, Supriyatno B, Darwin E, Basir D. Vitamin D status in under-five children with a history of close tuberculosis contact in Padang, West Sumatra. Asia Pac J Clin Nutr 2017;26(Suppl 1):S68–72.

50. Badan Pusat Statistik. Jumlah penduduk menurut kelompok umur dan jenis kelamin, 2022 [Internet]. Jakarta (Indonesia), Badan Pusat Statistik. 2021;[cited 2022 Jun 21]. Available from: https://www.bps.go.id/indikator/indikator/view_data_pub/0000/api_pub/YW40a21pdTU1cnJxOGt6dm43ZEdoZz09/da_03/1.
51. Jeyakumar A, Shinde V. A systematic review and meta-analysis of prevalence of vitamin D deficiency among adolescent girls in selected Indian states. Nutr Health 2019;25:61–70.



52. Mogire RM, Mutua A, Kimita W, Kamau A, Bejon P, Pettifor JM, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health 2020;8:e134–42.


53. Oktaria V, Putri DAD, Ihyauddin Z, Julia M, Sulistyoningrum DC, Koon PB, et al. Vitamin D deficiency in South-East Asian children: a systematic review. Arch Dis Child 2022;archdischild-2021-323765.


54. Torkaman M, Abolghasemi H, Amirsalari S, Beiraghdar F, Afsharpaiman S, Kavehmanesh Z, et al. Comparison of the vitamin D status of children younger and older than 2 years in Tehran: are supplements really necessary? Int J Endocrinol Metab 2016;14:e34676.



55. Odhaib SA, Alibrahim NT, Zaboon IA, Mansour AA. Vitamin D metabolic profiles in premenopausal women we aring niqab and hijab in sunny B asrah. Cureus 2021;13:e14909.



56. Alharbi AA, Alharbi MA, Aljafen AS, Aljuhani AM, Almarshad AI, Alomair IA, et al. Gender-specific differences in the awareness and intake of Vitamin D among adult population in Qassim Region. J Family Community Med 2018;25:148–54.



58. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin D Standardization Program (VDSP). Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40.

59. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJet al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons. 2019;pp 241–84.
60. Jacobs ET, Martínez ME, Jurutka PW. Vitamin D: marker or mechanism of action? Cancer Epidemiol Biomarkers Prev 2011;20:585–90.




61. Suaini NH, Koplin JJ, Ellis JA, Peters RL, Ponsonby AL, Dharmage SC, et al. Environmental and genetic determinants of vitamin D insufficiency in 12-month-old infants. J Steroid Biochem Mol Biol 2014;144 Pt B:445–54.


62. Angelin TC, Bardosono S, Shinta D, Fahmida U. Growth, dietary intake, and vitamin D receptor (VDR) promoter genotype in Indonesian school-age children. Nutrients 2021;13:2904.



63. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore) 2019;98:e17252.


64. Aji AS, Erwinda E, Rasyid R, Yusrawati Y, Malik SG, Alathari B, et al. A genetic approach to study the relationship b etween maternal Vit amin D st atus and ne w b orn anthropometry measurements: the Vitamin D pregnant mother (VDPM) cohort study. J Diabetes Metab Disord 2020;19:91–103.




65. Dhamayanti M, Noviandhari A, Supriadi S, Judistiani RT, Setiabudiawan B. Association of maternal vitamin D deficiency and infants' neurodevelopmental status: a cohort study on vitamin D and its impact during pregnancy and childhood in Indonesia. J Paediatr Child Health 2020;56:16–21.



66. Ariganjoye R. Pediatric Hypovitaminosis D: molecular perspectives and clinical implications. Glob Pediatr Health 2017;4:2333794X16685504.


67. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.


68. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18.










