Interpretation of androgen and anti-Mullerian hormone profiles in a Hispanic cohort of 5- to 8-year-old girls with premature adrenarche
Article information
Abstract
Purpose
Premature adrenarche (PA) often leads to polycystic ovary syndrome (PCOS). Higher anti-mullerian hormone (AMH) levels are reported in PCOS. We studied the androgen profile and AMH profiles in Hispanic girls with PA (aged 5–8 years) and age and body mass index (BMI) matched controls.
Methods
Retrospective review of electronic medical records of girls who met the inclusion criteria for premature adrenarche were done.
Results
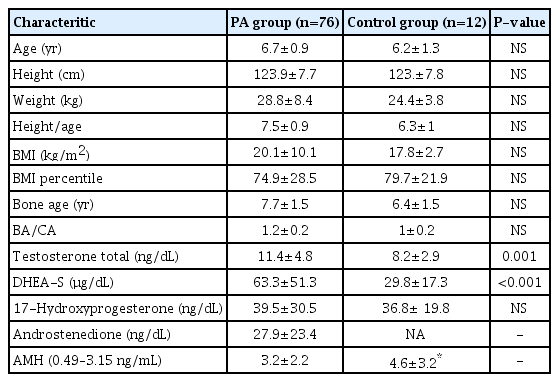
PA girls (n=76) were matched to control girls (n=12) for age (mean±standard deviation) (6.7±1 years vs. 6.2±1.3 years) and BMI (20±10 kg/m2 vs. 17.8±2.7 kg/m2). Dehydroepiandrostenedione sulfate (63.3±51.3 μg/dL vs. 29.8±17.3 μg/dL, P<0.001) and testosterone levels (11.4±4.8 ng/dL vs. 8.2±2.9 ng/dL, P=0.001) were significantly higher in the PA group than controls. AMH values (<14 years: reference range, 0.49–3.15 ng/mL) were 3.2±2.2 ng/mL vs. 4.6± 3.2 ng/mL respectively in the PA and control groups and were not different (P=0.4). AMH did not show a correlation with bone age (P=0.1), and testosterone (P=0.9) in the PA group. 17-hydroxyprogesterone levels (17-OHP ng/dL) were 39.5±30.5 ng/dL vs. 36.8±19.8 ng/dL in PA versus control girls. The concentration of 17-OHP was not statistically different between the control and PA groups.
Conclusions
Higher AMH was not observed in PA girls and no correlation with BA and androgen levels was observed.
Introduction
Premature adrenarche (PA) is the result of early activation of the zona reticularis with a rise of dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), androstenedione and testosterone (T) levels without a rise in cortisol. PA is the appearance of pubic hair (pubarche) and/or body odor in children between 5–8 years of age which is often associated with a rise in DHEAS [1]. Biochemical adrenarche is a rise of DHEAS level ≥1 μmol/L (≈40 μg/dL) whereas clinical adrenarche is the appearance of hyperandrogenism without elevation of androgens. Most girls with PA have normal levels of androgens [2,3] though; occasionally they can have values in the early pubertal range [4,5]. Even though, PA is known as a benign condition, it’s correlation with insulin resistance [6-8] and polycystic ovary syndrome (PCOS) has been reported [9-12].
There are several triggers for PA which include but are not limited to high BMI [13,14], ovarian hyper-responsiveness to adrenocorticotrophic hormone (ACTH) [6], history of being small for gestational age [15,16]. In vivo and In vitro studies support a role of the insulin/insulin-like growth factor-1 system in adrenarche [17] which also has been described in the pathophysiology and progression of PCOS [8]. Nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency is present in 7% children with premature pubarche [18].
Anti-Mullerian hormone (AMH) has been established to be a marker of ovarian follicle reserve, being secreted by preantral follicles (size, 2–5 mm). The preantral follicle number correlates with the elevated androgen levels seen in women with PCOS [19]. Two to 3 fold elevated AMH levels have been reported in women with PCOS [19]. Twenty percent of children with PA go on to develop PCOS [11]. Therefore, the question arises as to whether indices, such as AMH or ovarian morphology, might predict future risk for PCOS. We hypothesized that demonstration of higher AMH levels in girls with PA could serve as a predictor for PCOS.
In this retrospective study, we proposed to compare the androgen profile and bone age (BA) of Hispanic girls with PA to age-matched control girls and to evaluate AMH levels in Hispanic girls with PA.
Materials and methods
This study was a retrospective chart review study approved by the Institutional Review Board of Health and Hospital Corporation (Bellevue and Woodhull) and New York University School of Medicine. As no personal health information identifiers were used for data collection, the study was an exempt review by the Institutional Review Board and it conformed to the Health Insurance Portability and Accountability Act. The electronic medical records were reviewed by 2 investigators for patient charts coded as "premature adrenarche" and "premature pubarche" for the period between January 2002 and November 2015.
PA was defined as an appearance on exam of pubic or axillary hair, all girls being premenarchal. Exclusion criteria were: (1) <5 and >8 years in age, (2) signs of central puberty such as premature thelarche and menarche at the time of clinic visit, (3) peripheral precocious puberty, and (4) thyroid illness. Late-onset congenital adrenal hyperplasia was excluded by androgen precursor levels being markedly elevated and in these girls ACTH stimulation was done to establish NCAH using established norms. No adrenal or ovarian ultrasonography was performed. The control group included girls 5–8 years evaluated in our clinic with other endocrinologic disorders. They had androgen levels done for suspected adrenarche but androgen levels were normal for age. Data from 76 Hispanic girls’ cases and 12 age and BMI matched controls were analyzed.
Date of the first visit, medical history, body mass index (BMI) and BA were recorded using the Bayley-Pinneau method [20]. Weight and height were measured by using a conventional scale and stadiometer. BMI percentiles were assessed using age and sex-specific BMI reference [21]. BA/chronological age was a ratio and if >1 was consistent with BA advancement. Blood sampling was conducted at the time of the clinic visit though sampling was done at the same time of the day. Androgen levels and BAs were done as standard of care. AMH levels were done on stored samples which were stored at -80℃.
Radioimmunoassay (RIA) of testosterone and DHEAS used an organic solvent extraction and chromatography to eliminate interfering steroids to achieve high assay specificity. Patient serum samples were extracted with ether and subjected to celite partition chromatography using ethylene glycol as stationary phase and varying concentrations of ethyl acetate in isooctane as a mobile phase. Sensitivity of the assay for testosterone was 4 pg/tube and the mean intra- and interassay variation for T was 6.3% and 7.8%, respectively in the range of 5–30 pg/tube [22] (MP Biomedicals, Solon, OH, USA). RIA for DHEAS was directly performed on a highly diluted serum sample without extraction or purification. The sensitivity of the assay for DHEAS was 6 pg/tube with an intra- and interassay variation of 7.1% and 8.3%, respectively (MP Biomedicals). 17-OH progesterone was done by RIA after extraction and purification by liquid-liquid chromatography on celite. Antibody was from MP Biomedicals and the tritiated tracer used for competitive binding was from American Radiochemical (St. Louis, MO, USA). Androstenedione was done at Quest using liquid chromatography/Tandem Mass Spectrometry. AMH samples were analyzed by conventional Gen II Assay [23]. The test method has been described in previous reference [24]. The lowest amount of AMH in a sample that can be detected with a 95% probability was calculated to be 0.08 ng/mL and intra- and interassay coefficient of variation were 5.3%–11.4% and 3.8%–17.3%, respectively [25]. Hagen et al. [26] has reported the normal reference range for AMH for children and adolescents <14 years
Data were analyzed with IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). A P-value <0.05 was used to infer statistical significance. Kolgomorov-Smirnov and Shapiro-Wilks tests were used to examine the normality of distribution of the variables. For univariate analysis there were 2 groups: girls with PA and age-matched Hispanic girls. Anthropometric measurements (age, height, and BMI), BA, BA/chronological ratio (BA/CA), androgen profile (testosterone, DHEAS, 17-OH progesterone, and androstenedione) and AMH levels were compared between the 2 groups using 2-sample Student t-test or Wilcoxon rank sum test depending on the normality of distribution. Pearson or Spearman correlation coefficients were used to examine the linear or rank order relationship between variables of interest such as anthropometric data, BA, androgen profile, and AMH levels.
Results
PA girls (n=76) were matched to control girls (n=12) for age (mean±standard deviation [SD]) (6.7±0.9 years vs. 6.2±1.3 years) and BMI (20.1±10.1 kg/m2 vs. 17.8±2.7 kg/m2). BA and BA/CA at diagnosis were 7.7±1.5 years vs. 6.4±1.5 years and 1.2±0.2 years vs. 1±0.2 years, respectively (Table 1).
Testosterone levels (reference range for prepubertal girls: <10 ng/dL) were done in 66 of 76, DHEAS (5–8 years: reference range, 9–75 μg/dL) 68 of 76 and AMH (<14 years: reference range, 0.49–3.15 ng/mL) 33 of 76 of the girls with PA. In the control group testosterone, DHEAS were available for all girls, AMH could be done only in 6 of 12 girls. DHEAS (63.3±51.3 μg/dL vs. 29.8±17.3 μg/dL, P<0.001) and testosterone levels (11.4±4.8 ng/dL vs. 8.2±2.9 ng/dL; P=0.001) were different in the PA and control groups. Among the girls with PA, 17-OHP levels were reported in 68 of the 76 girls (39.5±30.5 ng/dL vs. 36.8±19.8 ng/dL, P=0.15) while androstenedione levels were reported in 30 of the 76 girls (27.9±23.4 ng/dL).
Testosterone levels were elevated above the established lab reference range for our RIA assay in 64% (42 of 66) of girls with PA compared to 25% of girls in the control group. DHEAS was elevated in 19% (13 of 68) of girls with PA compared to no girl in the control group. DHEAS correlated with height (r=0.3, P=0.03) and BA (r=0.3, P=0.02) (Fig. 1) using a Pearson correlation.

Pearson correlation (linear regression); P=0.006, r=0.034. DHEAS, dehydroepiandrostenedione sulphate.
AMH values were 3.19±2.24 (range, 0.068–8.8) and 4.7±2.7 ng/mL in the PA (n=33) and control group (n=6), respectively. AMH level did not show any association with either DHEAS (P=0.2), testosterone (P=0.9) or BA (P=0.1) in girls with PA.
Discussion
In this study, the DHEAS and testosterone were higher in PA girls compared to controls, but there were no differences in the 17-OHP between the 2 groups. DHEAS have been higher in girls with PA compared to normal timed girls [5,15]. while other studies have shown DHEAS levels being within reference range for age [27]. Dorn et al. [5] showed testosterone above detection limit in 56% of girls with PA compared to 23% in age-matched girls (P=0.004) using RIA methodology (most girls had values below the detection limit. Our results align with this group in that 65% our PA cohort had above lab reference testosterone levels. Uçar et al. [28] also demonstrated higher testosterone levels (45.1 pmol/L vs. 31.2 pmol/L, P=0.001) in Turkish girls with PA (n=56) when compared to control girls (n=33) using an RIA assay similar to ours. On the other hand, Paterson et al. [27] did not find elevated testosterone in Scottish girls when compared to normal reference range using a chemiluminescent assay (mean+SD [range]). Dorn et al. [5] reported mildly elevated androstenedione within the reference range, while elevated 17-OHP has not been reported in PA and more consistent with the diagnosis of NCAH. Elevation of testosterone in girls with PA may be to be driven by age, ethnicity (Hispanic vs. Turkish vs. White) and assay use. Age and ethnicity affect the age of onset of adrenarche as shown the National health examination study (Third National Health and Nutrition Examination Survey) [29]. Immunoassays like a sensitive RIA (as the one used by our Endocrine lab) have been compared to mass spectrometry and have good correlation for detection of low testosterone levels in prepubertal girls [30]. Longitudinal follow up in PA girls with elevated testosterone level on diagnosis may show to have predictive value for future development of PCOS.
Our results show that Hispanic girls with PA have minimal BA advancement, which is not different from age-matched controls. This result is in contrast to the result of previous studies that BA advancement was observed in PA children. Sopher et al. have shown that BA is often advanced in girls with PA and 30% will have advancement of >2 years [31-34]. DeSalvo et al. [33] showed that even though girls with PA were taller and heavier their final height was just above their midparental height and the authors speculated that the effects of an advanced BA on final height were minor.
Our results show that Hispanic girls with PA do not have elevated AMH levels. During childhood and adolescence, AMH fluctuations are minimal, and each girl maintains her relative level during pubertal transition. AMH levels start to rise by much as 17% three years before puberty and declines by 30% within the first 2 years of puberty. Several studies have reported higher AMH levels in women with PCOS [35-37]. Crisosto et al. [38] showed higher (AMH) levels in prepubertal and peripubertal daughters of PCOS women, suggesting that these girls may have an altered follicular development which starts early in development and continues through puberty. However, there is no correlation between the level of AMH before pubertal onset and age at entering puberty [26]. Since there are many studies that report a high incidence of PCOS in girls with PA, this study attempted to verify if the AMH level is higher in PA girls. One study with Scottish girls reports that the AMH level is higher in PA girls, and 3 studies including this study (Uçar, Utriainen, and this study) report that there is no difference in AML level between the 2 groups [27,28,39]. Two of the 3 studies reporting no difference in AMH levels (Ucar and this study) showed no difference in BMI between the 2 groups, but Utriainen’s study found a significant difference in the BMI between the 2 groups in and compared the AMH level while controlling the BMI. In the Scottish study, the difference in BMI cannot be verified because there is no detailed description of the anthropometric value of the control group [27]. Therefore, there seems to be no difference in the AMH level in prepubertal PA girls in a few studies that have been reported until now including this study. Additional research is necessary to assess to determine whether the AMH level gradually increases after the beginning of puberty in PA girls, and if it increases, whether this is correlated with the occurrence of PCOS later on.
We had several limitations in our study. First, it is a relatively small cohort with few controls; further studies with a larger population are needed in order to have a more precise conclusion regarding the association between AMH and PA. We also recognize that the AMH Gen II used in this study was the conventional and not revised assay which is more sensitive than the older version. Furthermore, the fact that our findings are of Hispanic girls brings into question the generalizability of our information. Our study is the first of its kind to study the AMH levels in the Hispanic ethnic cohort of girls with PA. We did match the PA girls for age and BMI controls and used sensitive assays for variables of interest. These are positive aspects of our study design.
In this study, we demonstrated that serum AMH was not elevated in prepubertal Hispanic girls with PA, and AMH levels were not associated with androgen profile or BA in prepubertal Hispanic girls. A longitudinal study on biochemical markers (serial AMH, androgens, gonadotropins) and pelvic ultrasonography is necessary for PA girls in pubertal ages before the start of menstruation as well as in prepubertal ages.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.