Thyrotoxic hypokalemic periodic paralysis due to Graves’ disease in 2 adolescents
Article information
Abstract
Thyrotoxic periodic paralysis (TPP) is a notable and potentially lethal complication of thyrotoxicosis, and Graves’ disease is the most common cause of TPP. TPP is commonly reported in Asian males between 20–40 years of age, but it is rare in children and adolescents. We report 2 Korean adolescents (a 16-year-old male and a 14-year-old female) with episodes of TPP who were previously diagnosed with Graves’ disease. These 2 patients presented with lower leg weakness in the morning after waking up. They were diagnosed with TPP-associated with thyrotoxicosis due to Graves’ disease. After they were initially treated with potassium chloride and antithyroid drugs, muscle paralysis improved and an euthyroid state without muscle paralytic events was maintained during follow-up. Therefore, clinicians should consider TPP when patients have sudden paralysis and thyrotoxic symptoms such as goiter, tachycardia, and hypertension.
Introduction
Thyrotoxic periodic paralysis (TPP) is an uncommon but serious complication of hyperthyroidism, and it is associated with hypokalemia [1]. TPP is characterized by recurrent muscle weakness and paralysis. Fatal complications, including ventricular arrhythmias, can occur in severe cases of TPP [2]. TPP mainly affects male patients of Asian descent, especially those 20–40 years of age, and the incidence has been reported at a rate of approximately 2% in hyperthyroidism patients [3]. The incidence of TPP in pediatric patients is very low compared to that in adult patients. Although it is rare in children and adolescents, some cases of TPP in adolescents have been reported from China, Korea, and in other ethnic groups [4]. Most recently, the reported cases of TPP have been attributable to Graves' disease, although autoimmune thyroiditis can be a very rare cause of TPP in children and adolescents. Here we report cases of 2 Korean adolescents who presented with TPP as clinical manifestation of Graves' disease along with a comprehensive literature review of pediatric patients in Korea.
Case reports
1. Case 1
A 16-year-old Korean boy had one episode of lower leg paralysis and upper extremity weakness in the morning after waking up and without having any symptoms associated with thyrotoxicosis. Prior to showing symptoms, the patient had practiced Japanese fencing for one hour the night before.
The patient did not have any past or family history associated with paralysis symptoms. He had a height of 168.3 cm, a weight of 69.4 kg, and a body mass index of 24.5 kg/m2. He had a grade 1 goiter based on World Health Organization criteria. When he arrived, his vital signs were stable with normal neurological examination findings, with the exception of motor weakness. His upper and lower extremity motor grades were 4 for both arms and 3 for both legs. His sensory responses were intact and his initial electrocardiogram and echocardiography results were normal.
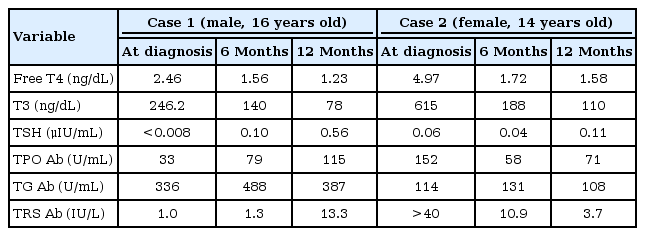
Laboratory findings showed normal complete blood cell counts and electrolytes with the exception of decreased potassium levels (K+, 2.5 mmol/L). A thyroid function test showed hyperthyroidism: serum free thyroxine (fT4), 2.46 ng/dL (range, 0.79–1.86 ng/dL), triiodothyronine (T3), 2.46 ng/mL (range, 0.76–1.90 ng/mL), and thyroid-stimulating hormone (TSH), < 0.008 IU/L (range, 0.15–5.0 IU/L). His serum thyroglobulin antibody (TG Ab) level was at 336 U/mL (range, 0–60 U/mL) and his thyroid peroxidase antibody (TPO Ab) level was 33 U/mL (range, 0–60 U/mL). However, his serum TSH receptor antibody level was 1.0 IU/L (range, 0–1.5 IU/L). Thyroid ultrasonography showed diffuse mild enlargement with heterogeneous echogenicity and prominent vascularity. A thyroid scan with technetium 99m pertechnetate showed that both lobes were normal in size with homogenous trapping. He was initially treated with potassium chloride (KCl), 1.1 mEq/kg/day intravenously for 4 hours, and oral potassium (K-contin), two 600-mg tablets once daily for 3 days, and 5 mg of methimazole once daily. We did not use a β-adrenergic blocker because he was asymptomatic for tachycardia based on the thyrotoxicosis symptoms. After 2 hours of loading KCl intravenously, his upper and lower extremity motor grades were 5 and 4, respectively. After 10 hours, his motor grades were all intact and his K+ level was normalized to 4.8 mmol/L. On hospital day 4, he was discharged with methimazole. At 1-year follow-up, his serum levels of TG Ab, TPO Ab, and TSH receptor antibodies were 482 U/mL, 134 U/mL, and 10.3 IU/L, respectively (Table 1). Based on these results, the patient was diagnosed with hypokalemic paralysis due to Graves' disease. Two years later, he is still taking methimazole 5 mg once a day, and maintaining an euthyroid status without muscle paralytic events.
2. Case 2
A 14-year-old female patient was admitted to the hospital through an outpatient clinic for treatment of tachycardia, palpitation, and thyroid hypertrophy. On admission, she had no symptoms of limb paralysis. However, on the following day, she complained of lower leg weakness in the proximal area in the morning after waking up. Unlike the first case, she was in a bed rest status to prevent worsening of thyrotoxicosis. Thus, she had not performed any significant physical activity before the onset of paralytic symptoms.
She had no past or family history associated with paralysis symptoms. Her height was 166.1 cm with a weight of 45.9 kg. Her body mass index was 16.64 kg/m2. At our hospital, her blood pressure was 164/96 mmHg with a body temperature of 36.6℃ and a heart rate of 136 beats/min. Exophthalmos and grade 2B goiter were confirmed on physical examination. Her neurological examination findings were normal except for motor weakness.
When she had symptoms of weakness, her upper and lower extremity motor grades were 5 and 4, respectively. However, her sensory responses were intact. Laboratory findings showed a serum potassium level of 3.1 mmol/L. There were no abnormalities of blood gas or inflammatory factors, including erythrocyte sedimentation rate and C-reactive protein. A thyroid function test showed hyperthyroidism, with a fT4 level at 4.97 ng/dL (range, 0.79–1.86 ng/dL), a T3 level at 6.15 ng/mL (range, 0.76–1.90 ng/mL), and a TSH level at 0.06 IU/L (range, 0.15–5.0 IU/L). Her serum TG Ab level was at 114 U/mL (range, 0–60 U/mL) and her TPO Ab level was at 152 U/mL (range, 0–60 U/mL) with a serum TSH receptor stimulating antibody level at >40 IU/L (range, 0–1.5 IU/L) (Table 1). Her initial electrocardiogram showed a sinus rhythm with a first degree atrioventricular block while her echocardiography results were normal. Thyroid ultrasonography showed an enlarged thyroid size and hypervascularity. A thyroid scan with technetium 99m pertechnetate showed diffuse enlargement of both lobes with increased trapping. These results indicated hypokalemic paralysis due to Graves' disease.
She was initially treated with KCl (0.348 mEq/kg, oral [K-contin], two 600-mg tablets 3 times) for 2 days, an antithyroid drug (prophylthiouracil) 150 mg 3 times a day (10 mg/kg/day) and a β-adrenergic blocker (propranolol 1 mg/kg/day) 15 mg 3 times a day (Table 2). Because she showed high fT4 and T3 levels with tachycardia, hypertension, and diarrhea, we determined that her condition was an imminent thyroid storm. Therefore, we prescribed propylthiouracil which can help inhibit peripheral conversion [5]. After 8 hours, her sensory and motor grades were all intact and her serum potassium level was normalized to 4.7 mmol/L. On hospital day 8, she was discharged with a β-adrenergic blocker (propranolol 15 mg 3 times a day) and an antithyroid drug (propylthiouracil 150 mg 3 times a day). At her 6-month follow-up, she maintained an euthyroid state without muscle paralytic events. Follow-up serum TG Ab and TPO Ab levels were 131 U/mL and 58 U/mL (range, 0–60 U/mL), respectively. Three years later, she is still taking an antithyroid drug. Thyroid hormone levels and thyroid-associated antibodies are constantly maintained at normal levels.
Discussion
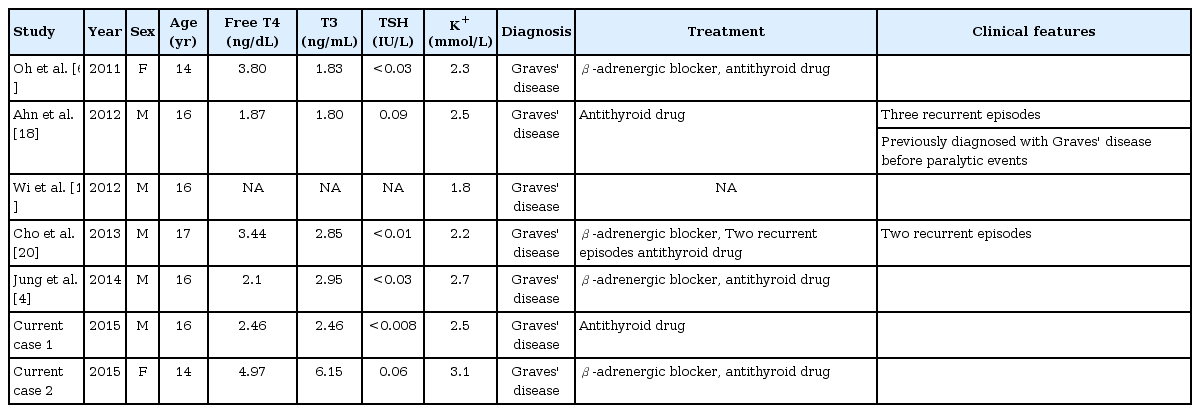
TPP is the most frequent form of acquired muscle paralysis in young adults [6]. The ratio of cases in males to females ranges from 17:1 to 70:1 [7]. TPP is common in Asia. To date, genes that have been studied in relation to TPP include the KCNJ18 and KCNJ2 genes. The KCNJ18 gene is known to be more common in Western countries, and has been reported at rates of 33.3% in Caucasian populations, primarily among individuals with Brazilian heritage, 25.9% in Singaporeans, and 1.2% in people from Hong Kong [8]. In contrast, in recent studies, genetic variants of the KCNJ2 gene have been reported to affect the development of TPP in Korean and Chinese populations [9,10]. These ethnic genetic differences might explain the difference in the incidence of TPP. The most common cause of TPP is Graves' disease. However, TPP can occur in any type of thyrotoxicosis [6]. In general, autoimmune thyroid diseases such as Graves’ disease are more common in pubertal girls than boys. However, the incidence of TPP is higher in boys than in girls [7]. Reported TPP cases in Korean adolescents have been primarily males (5 males and 2 females) and all patients had been diagnosed with Graves' disease (Table 2). Six patients had elevated serum thyroid hormone levels and they were treated with methimazole and/or a β-adrenergic blocker. Among previously reported cases, two patients had recurrent paralytic episodes during antithyroid drug treatment.
Hypokalemia in TPP develops by a rapid and massive shift of potassium from the extracellular to the intracellular compartment, mainly into muscles. Thyroid hormone can stimulate Na+–K+ ATPase in skeletal muscle by transcriptional up-regulation of the gene that encodes Na+–K+ ATPase and by enhancing the intrinsic activity or membrane insertion of the pump [11]. Thus, there is no pure potassium depletion in the body [12]. Insulin, a high carbohydrate meal, and exercise are other well-known activators of Na+–K+ ATPase [13]. Although serum potassium levels are usually decreased in TPP, as in our reported cases, in exceptionally rare circumstances normal serum potassium levels can lead to normokalemic TPP. Patients who initially showed normokalemic TPP also eventually progressed to hypokalemia after [14]. However, the pathogenesis of normokalemic TPP remains unclear.
Diagnosis of TPP is based on recurrent clinical features of TPP and blood test results suggestive of hyperthyroidism and hypokalemia. The severity of paralysis is correlated with the degree of hypokalemia, but not with clinical signs/symptoms of hyperthyroidism or thyroid hormone levels [7]. Previously reported cases with TPP had only mildly elevated serum thyroid hormone levels. Ko et al. [15] have reported that only 10% of patients have mild thyrotoxic symptoms. Therefore, TPP should be distinguished from other causes of acute paralysis such as familial hypokalemic periodic paralysis, Guillain-Barré syndrome, myasthenic crisis, and conditions that produce spinal cord compression [16]. TPP is very rare in children and adolescents and is even more unusual in girls. In addition, although there are aggravating factors, such as a high carbohydrate meal and exercise, that are associated with the occurrence of TPP, TPP can occur without such factors as was seen in our second case. Therefore, based on the two cases in this report, the occurrence of TPP should always be considered regardless of sex when treating patients with Graves' disease.
Treatment of TPP includes correction of hypokalemia and maintenance of an euthyroid status. Immediate supplement with KCl is needed to prevent major cardiopulmonary complications and to foster muscle paralysis recovery [12]. However, another study has reported that there is lack of correlation between recovery time of muscle paralysis and the dose of infused KCl [17]. Despite intravascular hypokalemia, the total body potassium level is normal. Thus, KCl infusion should be administered with caution. KCl infusion causes rebound hyperkalemia in up to 70% of cases, and as a result, fatal arrhythmias may occur. Rebound hyperkalemia can be prevented by lower doses of KCl and close cardiac monitoring is necessary [16]. A β-adrenergic blocker is an alternative treatment that can reduce paralysis without rebound hyperkalemia. Because TPP does not usually recur once an euthyroid state has been attained, adequate control of hyperthyroidism is important after the acute phase [7]. Currently, antithyroid drugs remain the mainstay treatment of hyperthyroidism.
In conclusion, TPP is a very rare muscle disorder in children and adolescents. Any etiology of hyperthyroidism can be associated with TPP, including Graves’ disease, Hashimoto’s thyroiditis, and toxic nodular adenoma. Thus, pediatricians should perform thyroid function tests when treating patients with sudden paralysis and thyrotoxic symptoms including goiter, tachycardia, and exophthalmos.
Notes
Ethical statement: This study was approved by the Institutional Review Board of the Ajou University Hospital (AJIRB-MED-EXP-18-250). The requirement for informed consent was waived due to the retrospective nature of this study.
Conflict of interest: No potential conflict of interest relevant to this article was reported.