Metabolic syndrome induced by anticancer treatment in childhood cancer survivors
Article information
Abstract
The number of childhood cancer survivors is increasing as survival rates improve. However, complications after treatment have not received much attention, particularly metabolic syndrome. Metabolic syndrome comprises central obesity, dyslipidemia, hypertension, and insulin resistance, and cancer survivors have higher risks of cardiovascular events compared with the general population. The mechanism by which cancer treatment induces metabolic syndrome is unclear. However, its pathophysiology can be categorized based on the cancer treatment type administered. Brain surgery or radiotherapy may induce metabolic syndrome by damaging the hypothalamic-pituitary axis, which may induce pituitary hormone deficiencies. Local therapy administered to particular endocrine organs directly damages the organs and causes hormone deficiencies, which induce obesity and dyslipidemia leading to metabolic syndrome. Chemotherapeutic agents interfere with cell generation and growth, damage the vascular endothelial cells, and increase the cardiovascular risk. Moreover, chemotherapeutic agents induce oxidative stress, which also induces metabolic syndrome. Physical inactivity caused by cancer treatment or the cancer itself, dietary restrictions, and the frequent use of antibiotics may also be risk factors for metabolic syndrome. Since childhood cancer survivors with metabolic syndrome have higher risks of cardiovascular events at an earlier age, early interventions should be considered. The optimal timing of interventions and drug use has not been established, but lifestyle modifications and exercise interventions that begin during cancer treatment might be beneficial and tailored education and interventions that account for individual patients' circumstances are needed. This review evaluates the recent literature that describes metabolic syndrome in cancer survivors, with a focus on its pathophysiology.
Introduction
The incidence of childhood cancer in South Korea has gradually increased, because detection and diagnostic technologies have improved1). The childhood cancer patient survival rate has also improved to over 78%1), because of improvements in the treatment modalities and supportive care. Hence, there are more adult childhood cancer survivors who are experiencing the delayed effects of cancer treatment and whose quality of life is compromised. Among the complications associated with cancer treatment, metabolic syndrome is an important delayed manifestation that is strongly associated with the quality of life. Metabolic syndrome comprises a cluster of cardiovascular risk factors, namely, central obesity, insulin resistance, dyslipidemia, and hypertension2). The increased risk of metabolic syndrome among childhood cancer survivors was first noted in 19963), and increased rates of metabolic syndrome and cardiovascular risk factors among cancer survivors have been reported subsequently245). In South Korea, investigations into metabolic syndrome among childhood cancer survivors have been limited, because they have comprised single center studies or involved small numbers of patients6789). This review evaluates the recent literature that describes metabolic syndrome in cancer survivors, with a focus on its pathophysiology.
Characteristics of metabolic syndrome in cancer survivors
Early studies of metabolic syndrome in cancer survivors focused on the tendency of children who had survived cancer treatment to be obese1011), then, the patterns of glucose metabolism, insulin resistance, the changes in growth hormone (GH) secretion, and the lipid profiles were analyzed. Survivors of childhood acute lymphoblastic leukemia (ALL) tend to have greater risk of obesity3711), glucose intolerance12), lipid abnormalities913), and cardiovascular events14) compared to equivalently aged members of the general population. Table 1 summarizes the possible mechanisms underlying metabolic syndrome development that induced by different cancer treatments.
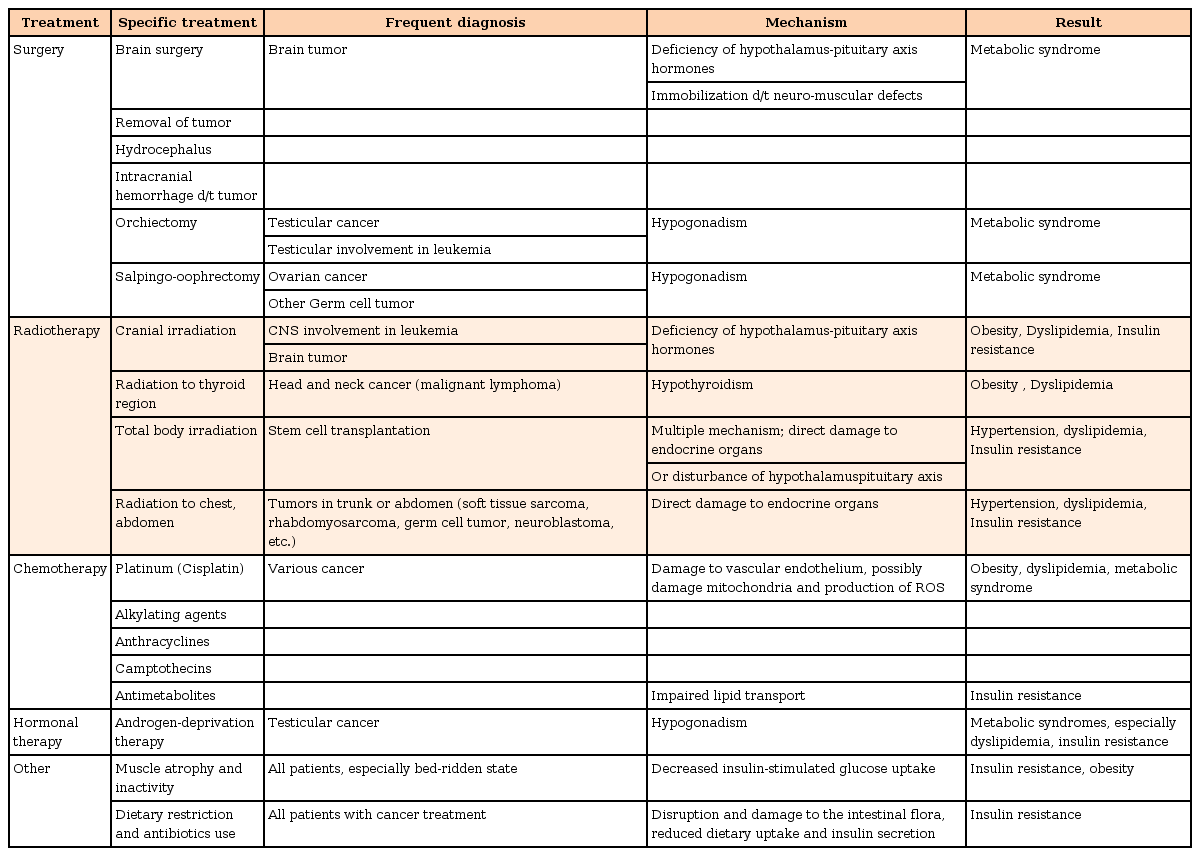
Possible mechanisms underlying metabolic syndrome development that is induced by different cancer treatments
1. Obesity
Obesity is the main contributor to metabolic syndrome development. Excessive amounts of free fatty acids lead to insulin resistance and glucose intolerance, and the adipose tissue is the main source of a variety of proinflammatory and prothrombotic cytokines that may contribute to the development of atherosclerotic cardiovascular changes2).
The mechanism underlying the higher risk of obesity among cancer survivors is unclear. However, the findings from the St. Jude cohort studies indicate that childhood ALL survivors tend to have hypertension and higher fasting glucose and cholesterol levels, specifically, high triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels14). Moreover, almost half of the survivors had body mass indices (BMIs) that were over 30 kg/m2. Risk analyses have shown that being male and Caucasian, and prior cranial irradiation with or without craniospinal irradiation are important risk factors associated with metabolic syndrome514). More recently, radiation- or chemotherapeutic agent-induced GH deficiency (GHD) has been determined to be the most important cause of obesity in cancer survivors15).
2. Dyslipidemia
Dyslipidemic features, including hypertriglyceridemia, high LDL-C levels, and low high-density lipoprotein cholesterol (HDL-C) levels, have been described in cancer survivors131617). The findings from many well-controlled and well-designed studies with few variables have demonstrated that cancer survivors are more dyslipidemic than control population. Since this cannot be explained by distinct factors, the genetic risk factors for obesity and dyslipidemia among adult childhood cancer survivors has been evaluated.
The features of dyslipidemia are closely related to cardiovascular risk. If the treatment modalities patient received are cardiotoxic, for example, anthracyclines, chest radiation, and hematopoietic stem cell transplantation, the patient will have a greater risk of cardiovascular events1418). Hence, our attention should focus on the timing of care initiation for childhood cancer survivors.
3. Genetic factors
Ross et al.19) reported that leptin receptor gene polymorphisms may influence obesity in female childhood ALL survivors, particularly those who had undergone cranial irradiation. Wilson et al.20) reported the results from a genome-wide association analysis of patients who had survived ALL treatment, and found several single nucleotide polymorphisms associated with obesity and cranial irradiation. Other attempts to find genetic differences among childhood leukemia survivors are ongoing51721).
Pathophysiology related to anticancer treatment
1. Surgery
Surgery can induce endocrine deficiencies by directly damaging the endocrine organs. Removing brain tumors can damage the hypothalamic-pituitary axis, which causes several hormonal and metabolic changes, and, eventually, metabolic syndrome. Orchiectomies, thyroidectomies, and oophorectomies induce sex hormone deficiencies, and the replacement of these hormones is an important factor associated with metabolic syndrome development22).
2. Radiotherapy
Radiotherapy causes direct organ damage in a dose-dependent manner. Resistance to radiation differs according to the organ undergoing treatment. Regarding the brain, isolated GHD can be induced by doses of 18–24 Gy, which are frequently used for cranial irradiation in patients with ALL with central nervous system involvement23). Doses of 30–50 Gy, which are usually used in patients with average-risk brain tumors, can permanently damage the gonadotropin, adrenocorticotropin, and thyrotropin axes, and doses that are higher than 60 Gy, that is, “full-dose radiation,” cause panhypopituitarism23), and these radiation doses damage other neurocognitive functions, thereby preventing patients from having ordinary “healthy” lifestyles. Patients who undergo hematopoietic stem cell transplantations with total body irradiation (TBI) as a conditioning regimen have higher diabetes and cardiovascular risks18).
3. Chemotherapy
While the exact role that chemotherapy plays in contributing to metabolic syndrome is unclear, chemotherapy is thought to induce metabolic syndrome, in part, by inducing gonadal hormone deficiencies2). Alkylating agents, anthracyclines, camptothecins, epipodophyllotoxins, and platinum-based agents are frequently used to treat pediatric cancers, and they disrupt DNA replication and transcription, and protein synthesis, thereby interrupting cell regeneration and growth. Endocrine cells might be more sensitive to the injury caused by these agents than other cells4). Moreover, these agents might interact with receptors or secondary messengers, thereby disrupting the hormonal axes4).
Chemotherapeutic agents induce insulin resistance, hyperinsulinemia, and impaired glucose control by directly influencing insulin sensitivity24). Alkylating agents, anthracyclines, camptothecins, epipodophyllotoxins, and platinum-based agents produce reactive oxygen species that mediate the anticancer effects, which lead to mitochondrial dysfunction15). Anemia, apoptosis, and cell lysis may lead to tissue hypoxia that causes the release of proinflammatory cytokines and macrophage activation15). All of these effects could contribute to the development of obesity, insulin resistance, and dyslipidemia, and, ultimately, metabolic syndrome.
4. Hormone therapy
The hormone-modifying therapeutic agents used in prostate cancer and breast cancer are associated with an increased risk of metabolic syndrome2224). These agents increase the LDL-C and TG levels, and insulin resistance, which leads to poor glycemic control in a prediabetic state. The cardiovascular and thromboembolism risks also increase following treatment with these agents2526). Fortunately, these agents are rarely used to treat pediatric cancer. However, risk of hormone or similar agents should be aware of for many clinicians caring cancer survivors.
Hormonal changes after anticancer treatment
Injuries to the hypothalamic-pituitary axis may cause somatotropin, thyrotropin, gonadotropin, and adrenocorticotropin imbalances, thereby causing specific hormonal deficiencies. Hormonal problems, especially those involving GH, thyroid hormones, and sex hormones are associated with metabolic syndrome2). While cranial radiotherapy clearly disrupts the hypothalamic-pituitary axis dose dependently23), the effects of chemotherapy on the hypothalamus and pituitary gland require further investigation.
1. Growth hormone deficiency
The impact of GHD on the height of childhood cancer survivors is well established. In addition to stimulating growth, GH stimulates protein synthesis, contributes to lipolysis, and it indirectly exerts insulin-like effects that include stimulating the uptake of glucose by the peripheral tissues by stimulating insulin-like growth factor-1 production by the liver and local tissues. Thus, GHD changes the body's composition by, for example, increasing the fat mass and causing obesity, which are associated with dyslipidemia and insulin resistance67).
Cranial radiotherapy is a major risk factor for obesity, dyslipidemia, and insulin resistance in childhood cancer survivors517). Hence, cranial radiotherapy may induce GHD, which may cause metabolic syndrome development. Since cranial radiotherapy used less frequently to treat childhood ALL and other cancers nowadays, the incidence of secondary GHD might decline. However, GHD has been reported in patients who did not undergo cranial radiotherapy5), which suggests that chemotherapy can also damage the hypothalamic-pituitary axis, but the mechanism underlying this damage is unknown.
2. Thyroid hormone deficiencies
The thyroid hormones are important for regulating the metabolism, and hypothyroidism and even a low-level euthyroid state are closely associated with metabolic syndrome that manifests as an increased waist circumference, increased TG and fasting glucose levels, and decreased HDL-C levels27).
Hypothyroidism is frequently seen after radiotherapy around the thyroid gland. The 5-year risk for hypothyroidism varies from 20% to 48% in patients with head and neck cancer who undergo radiotherapy28). Cranial irradiation and TBI associated with hematopoietic stem cell transplantations can cause primary and secondary hypothyroidism29). However, the findings from some studies demonstrate that patients who receive chemotherapy only show a high prevalence of subclinical hypothyroidism230). Therefore, knowledge about chemotherapy-induced hypothyroidism remains limited.
3. Gonadotropin and sex hormone deficiencies
Low testosterone levels are associated with visceral obesity, insulin resistance, and dyslipidemia, and, therefore, an increased risk of metabolic syndrome development2). Estrogen deficiencies are associated with central obesity, dyslipidemia, and insulin resistance24). Testosterone deficiencies in cancer survivors can be caused by orchiectomy and by direct damage from radiotherapy or chemotherapy with alkylating agents and heavy metals. Estrogen deficiencies in cancer survivors are usually caused by oophorectomy and by direct damage from radiotherapy or chemotherapy. One study's findings showed that low estrogen levels induced by bilateral oophorectomies were associated with an increased prevalence of metabolic syndrome31). In addition, effects of radiotherapy or gonadotropin-releasing hormone agonists used as anticancer treatment on the hypothalamic-pituitary axis might cause secondary gonadal dysfunction. Cranial radiotherapy for brain tumors or ALL is associated with secondary gonadal dysfunction in childhood cancer survivors31).
Changes in energy metabolism after anticancer treatment
Mayer et al.32) showed reduced levels of energy expenditure in childhood ALL survivors treated with cranial radiotherapy, which were thought to be caused by damage to the hypothalamic-pituitary axis and the development of GH and thyroid hormone deficiencies. This damage might also induce hypothalamic resistance to leptin's negative feedback effect on energy intakes and its positive feedback effect on energy expenditure. Therefore, childhood ALL survivors could be at an increased risk of becoming obese33). Cancer survivors who underwent hematopoietic stem cell transplantations showed similar manifestations31).
The mechanisms by which cancer treatments other than radiotherapy induce metabolic syndrome are unclear. Direct toxicity could lead to dysfunctional adipose tissues in cancer survivors, thereby contributing to body composition changes, insulin resistance, and the increased production of proinflammatory adipokines.
Other sequelae of anticancer treatment
Treatment-related neurotoxicity might induce autonomic dysfunction. Impairment of the arterial baroreflex is associated with an increase in sympathetic nervous activity and metabolic syndrome development2). Furthermore, specific complications caused by surgery or other anticancer treatments might make patients physically disabled. Patients with osteosarcomas, Ewing sarcomas, and brain tumors have noticeably reduced physical performance levels compared with patients with other cancers34). These complications can limit a patient's physical activity levels, thereby increasing the risk of metabolic syndrome.
Cancer survivors' lifestyle factors
Lifestyle factors, including exercise, diet, consuming alcohol, and smoking, affect the risk of developing metabolic syndrome. Cancer-treatment-related lifestyle changes caused by somatic complications or psychological factors, including stress and health consciousness, are important factors associated with the quality of life. Childhood cancer survivors are less physically active than their siblings, and physical, neurocognitive, and cardiorespiratory restrictions are important contributing factors34).
Studies into intervention and prevention
Cancer survival comprises follow-up assessments to detect relapses, counsel patients to maintain good health, and manage complications associated with cancer treatments. Pediatric oncologists and endocrinologists who manage cancer survivors should be aware of the potential effects of cancer treatment, including its delayed cardiometabolic effects and metabolic syndrome. While strategies have been established to manage metabolic syndrome in the general population, applying these guidelines to cancer survivors remains controversial.
1. Lifestyle modifications
Treating metabolic syndrome consists of lifestyle interventions with or without drug therapy35). Smoking cessation, lowering cholesterol levels, especially the LDL-C and TG levels, dietary interventions, exercise, and blood pressure management are the primary targets. Lifestyle interventions are initially recommended for metabolic syndrome, but if these are inadequate, drug therapies for the abnormalities associated with the individual risk factors may be indicated. According to the current guidelines, cardiovascular risk estimates and decisions about initiating drug treatment are largely based on age, and only 10-year risks are predicted24). However, childhood cancer survivors tend to be young when problems arise; hence, the guidelines are insufficient for younger cancer survivors36).
2. Exercise intervention
Although data describing the long-term effects of lifestyle interventions on cancer survivors are scarce, the findings from a recent short-term study of lifestyle interventions that mainly comprised exercise are promising. Bao et al.37) evaluated the impact of exercise in a population-based prospective cohort study of 1,696 breast cancer survivors and showed that exercise was inversely associated with the prevalence of metabolic syndrome, and that regular exercise participation reduced its prevalence. Similarly, Grote et al.38) studied the effects of exercise on patients who had survived different types of cancer, and their findings showed that a 3 day/week, 13-week exercise intervention improved the cardiometabolic health of cancer survivors. These investigators noted that the intervention was less effective in non-Caucasian survivors compared with Caucasian survivors, which warrants undertaking larger, multiethnic studies. However, no study reports describe the effects of lifestyle modifications on childhood cancer survivors.
3. Dietary and nutritional interventions
Cancer patients have poor health statuses, because of cachexia that develops during treatment and immobilization. However, few studies have focused on cancer survivors' nutrition and their health posttreatment. Tonorezos et al.39) analyzed the relationship between nutrition and metabolic syndrome in 117 adult survivors of childhood ALL, and showed that those who ate a Mediterranean diet had a lower prevalence of metabolic syndrome. Smith et al.40) reported that adhering to the so-called “good lifestyle and nutrition” habits recommended by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines was associated with a low risk of metabolic syndrome in the St. Jude Lifetime cohort. The WCRF/AICR guidelines recommend maintaining a BMI below 25 kg/m2, daily physical activity, daily fruit and vegetable intakes of over five servings/day, daily complex carbohydrate intakes of over 400 g/day, reducing daily alcohol intakes to below 14 g/day for women and 28 g/day for men, reducing daily red meat intakes to below 80 g/day, and reducing sodium intakes to below 2.4 g/day. However, 87.8% of the men and 87.2% of the women in the St. Jude Lifetime cohort did not follow the WCRF/AICR guidelines, and nearly 40% of the men and women showed the characteristics associated with metabolic syndrome.
A recent Cochrane review analyzed three randomized controlled studies of cancer survivors' nutrition41). Two studies' findings showed that interventions comprising calcium and vitamin D intakes did not influence bone cancer survivors' bone mineral densities. The third study's findings showed that cancer survivors tend to eat more junk food than the general population. The Cochrane review concluded that these studies failed to demonstrate the effects of nutritional interventions and that to elucidate the role of nutritional interventions on metabolic syndrome; further well-designed controlled studies are needed.
To date, no randomized controlled studies have been undertaken to investigate the impact of nutritional interventions on metabolic syndrome in childhood cancer survivors. Nevertheless, it seems reasonable to advise patients to follow the lifestyle and nutrition recommendations to reduce metabolic syndrome and cardiovascular event risks.
4. Optimal timing of interventions and education
The timing of lifestyle interventions is important. Lifestyle interventions usually begin after cancer treatment is completed. Nevertheless, whether there is a difference between exercising during or after cancer treatment and the extent of any difference remain unknown.
Weight gains start during therapy and obesity persists at the end of treatment, irrespective of whether patients undergo cranial radiotherapy5), which suggests that the risk of developing metabolic syndrome starts to increase during cancer treatment. Thus, initiating lifestyle modifications as early as possible, that is, during cancer treatment, would, theoretically, be more helpful at preventing weight gains and reducing the cardiovascular risk. If a patient exercises during cancer treatment, they can maintain a healthy weight and body composition, and prevent metabolic syndrome development. In addition, by including these lifestyle modifications during cancer treatment, these changes will be incorporated into the rest of the patients' lives, thereby helping to improve the quality of life24).
However, exercising during cancer treatment might be impossible for some patients, because of their clinical courses, cancer treatment intensities, and the severities of the cancers themselves. Hence, the timing and modes of lifestyle modifications and interventions should be customized to each patient's needs. Health care providers should prepare effective exercise and dietary intervention programs. Education and emphasizing the importance of lifestyle modifications should begin at the time of the cancer diagnosis and treatment.
5. Medical treatment
The current metabolic syndrome management guidelines recommend appropriate pharmaceutical management if lifestyle modifications do not improve the manifestations of metabolic syndrome35). The guideline parameters for drug therapy for dyslipidemia, hypertension, and elevated plasma glucose levels are based on specific coronary heart disease risk categories in adults that are derived from large, long-term observational studies, and no randomized controlled or long-term cohort studies of cancer survivors have been undertaken. Cancer survivors should be considered to have a specific cardiovascular risk that is posed by cancer treatment; therefore, their pharmaceutical management should be tailored to their individual needs.
Replacing GH might be possible for cancer survivors with GHD. Follin et al.42) reported that young adult childhood ALL survivors with GHD showed significant decreases in their serum leptin levels, leptin levels per kg fat mass, plasma glucose levels, and their waist and hip circumferences after 12 months of GH replacement therapy. However, no randomized studies of GH replacement therapy in childhood cancer survivors have been undertaken. The results from the Genetics and Neuroendocrinology of Short Stature International Study and the Hypopituitary Control and Complications Study43) demonstrated that secondary neoplasms in childhood cancer survivors administered GH replacement therapy occurred in 3.8% of children and in 6.0% of adults, and that the estimated cumulative frequencies of secondary neoplasms after 5 years were 6.2% and 4.8%, respectively. Further studies are needed to determine the efficacy and safety of GH replacement therapy in childhood cancer survivors who have GHDs induced by cancer treatments.
Conclusions
Childhood cancer survivors experience many problems after cancer treatment, but one of the most important complications is metabolic syndrome. Much remains to be discovered about the pathophysiology underlying metabolic syndrome in cancer survivors, including the genetic and environmental factors. Clearly, the cardiovascular risk caused by metabolic syndrome in cancer survivors is considerably higher than that in the general population. Childhood cancer survivors will increase in number as overall survival improves. Hence, to manage these children and adults effectively, the coordinated efforts of oncologists, endocrinologists, rehabilitation physicians, and other health care providers are required. This review may guide the next steps for further studies, and provide insights that help to evaluate and effectively manage high-risk childhood cancer survivors.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
