Etiology and therapeutic outcomes of children with gonadotropin-independent precocious puberty
Article information
Abstract
Purpose
This study was performed to investigate the etiology, clinical features, and outcomes of patients with gonadotropin-independent precocious puberty (GIPP).
Methods
The study included 16 patients (14 female and 2 male patients) who manifested secondary sexual characteristics, elevated sex hormones, or adrenal androgens with prepubertal luteinizing hormone levels after gonadotropin releasing hormone stimulation diagnosed between May 1994 and December 2015. Patients with congenital adrenal hyperplasia were excluded. Clinical features, laboratory findings, treatment modalities, and outcomes were retrospectively reviewed.
Results
The median age at diagnosis was 2.6 years (range, 0.7–7.9 years) and median follow-up duration was 4.6 years (range, 1 month–9.8 years). Patients with McCune-Albright syndrome (n=5) and functional ovarian cysts (n=4) presented with vaginal bleeding and elevated estradiol levels (23.3±17.5 pg/mL); adrenocortical tumors (n=4) with premature pubarche and elevated dehydroepiandrosterone sulfate levels (87.2–6,530 µg/dL); and human chorionic gonadotropin (hCG)-producing tumor (n=1) with premature pubarche and elevated β-human chorionic gonadotropin levels (47.4 mIU/mL). Two patients were idiopathic. Six patients transited to gonadotropin-dependent precocious puberty median 3.3 years (range, 0.3–5.1 years) after the onset of GIPP. Initial and follow-up height standard deviation scores (0.99±0.84 vs. 1.10±1.10, P=0.44) and bone age advancement (1.49±1.77 years vs. 2.02±1.95 years, P=0.06) were not significantly different.
Conclusion
The etiologies of GIPP are heterogeneous, and treatment and prognosis is quite different according to the etiology. Efficacy of treatment with aromatase inhibitors needs to be evaluated after long-term follow-up.
Introduction
Precocious puberty is defined as the development of secondary sexual characteristics before the age of 8 years in girls and 9 years in boys1). Precocious puberty is clinically categorized into gonadotropin releasing hormone (GnRH)-dependent and GnRH-independent processes according to the existence of hypothalamic-pituitary-gonadal axis activation. Central or gonadotropin-dependent precocious puberty (GDPP) results from activation of the hypothalamic-pituitary-gonadal axis by a variety of central nervous system abnormalities23).
Peripheral or gonadotropin-independent precocious puberty (GIPP) is caused by excessive sex hormones produced in the gonads or adrenal glands, β-human chorionic gonadotropin (β-hCG)-secreting tumors, or exposure to exogenous sex hormones. The common etiologies of excessive production of sex hormones by the gonads include McCune-Albright syndrome (MAS), functional ovarian cysts (FOCs), Leydig cell tumors, or familial male-limited precocious puberty. Adrenal origin of excess androgen production is caused by androgen-secreting tumors or congenital adrenal hyperplasia34). Treatment of GIPP is directed at the underlying pathology. However, in MAS or familial male-limited precocious puberty, treatments using aromatase inhibitors and antiestrogens or antiandrogens have demonstrated partial efficacy in reducing the growth rate and improving predicted adult height567).
GIPP is much less common than GDPP, regardless of the etiology. GDPP has a predictable physiologic and clinical course, whereas the pathophysiology and clinical manifestations of GIPP are heterogeneous according to its etiology. Appropriate treatment modalities and their efficacy in GIPP are obscure, and the long-term prognosis also remains uncertain. Therefore, this study aimed to determine the etiology, clinical features, and treatment outcome of patients with GIPP according to each etiology.
Materials and methods
1. Patients
This study included 16 patients with GIPP, who were diagnosed between May 1994 and December 2015 at Asan Medical Center Children's Hospital, Seoul, Korea. Fourteen female patients initially presented with breast or pubic hair development before the age of 8 years, or vaginal bleeding before the age of 9.5 years. Two male patients presented with penile enlargement or pubic hair development before the age of 9 years. Patients with congenital adrenal hyperplasia were excluded from the study because the incidence of precocious puberty in children congenital adrenal hyperplasia is much higher than other causes of GIPP, and clinical features were heterogeneous according to the phenotypes, age at diagnosis, and treatment.
2. Methods
The patients' clinical parameters, such as presenting symptoms, etiology, laboratory and radiologic findings, treatment, and clinical course, were reviewed retrospectively.
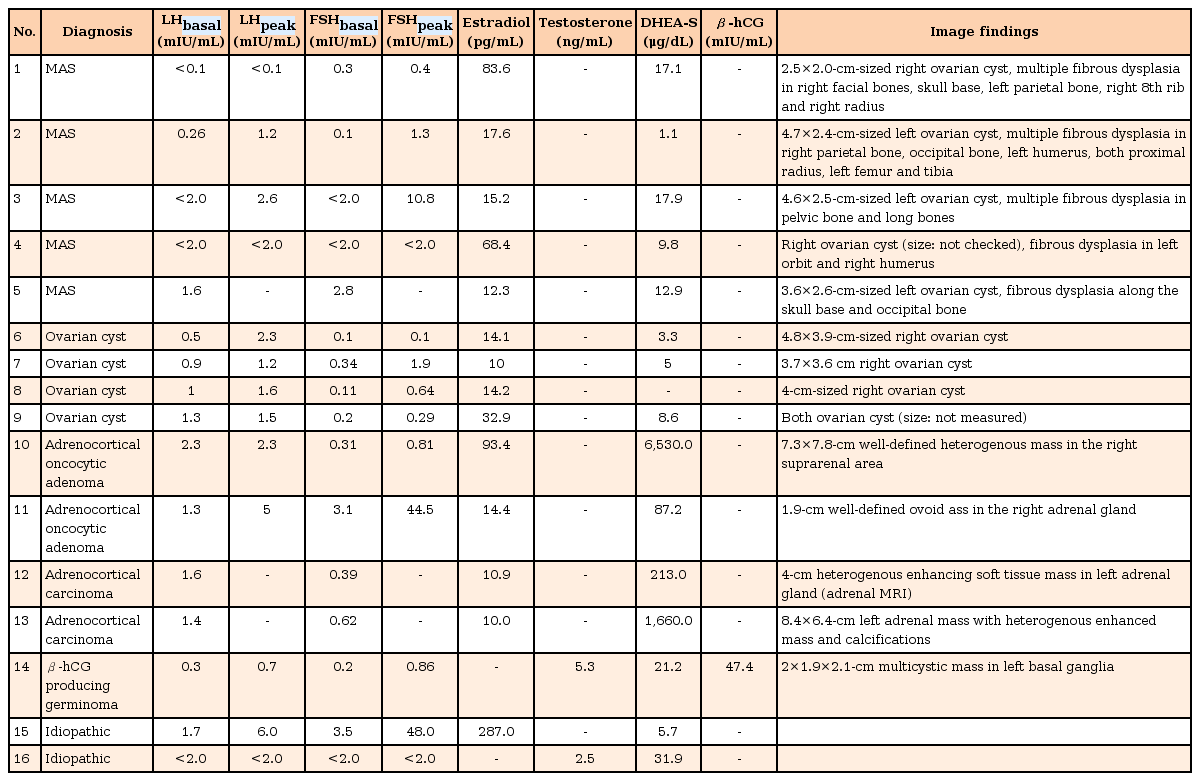
Basal luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (in females), testosterone (in males or females with pubarche), dehydroepiandrosterone sulfate (DHEA-S, in patients with pubarche) and β-hCG (in males) were measured in patients who presented with breast or pubic hair development.
Pelvic ultrasonography was performed in female patients with elevated estradiol levels. Adrenal ultrasonography or computed tomography was carried out in patients with pubic hair development or elevated DHEA-S levels. Tc-99m 3,3-diphosphono-1,2-propanedicarboxylic acid bone scan was done to investigate fibrous dysplasia in patients with ovarian cysts andcafé-au-lait spots. The screening for GDPP were performed when the patients revealed accelerated linear growth or advanced bone age (advanced bone age of at least 1 year relative to chronologic age).
The sex hormone or adrenal androgen levels were elevated in the patients, and the peak LH level was less than 5 mIU/mL after the stimulation of intravenous GnRH (Relefact, Sanofi-Aventis, Frankfurt am Main, Germany) at a dose of 2.5 µg/kg (maximum 100 µg). The diagnosis of GDPP were made when the peak LH level was higher than 5 mIU/mL after the stimulation of intravenous GnRH3).
Height was expressed as standard deviation score (SDS) according to the normative data from Korean references8). LH (LHsp-IRMA, BioSource Europe S.A, Nivelles, Belgium), FSH (FSH-IRMA, BioSource Europe S.A,), and β-hCG (RIAKEY, Shin Jin Medics Inc, Goyang, Korea) were measured with immunoradiometric assays. DHEA-S, estradiol (in females), and testosterone (in males or females with pubic hair development) were measured with radioimmunoassays (Coat-A-Count, Diagnostic Products Corp., Los Angeles, CA, USA). The intraassay coefficients of variation for LH were 3.9% and 1.4% for the mean concentration of 6.6 mIU/mL and 46.6 mIU/mL, respectively. The interassay coefficients of variation for LH were 8.0% and 3.4% for the mean concentration of 5.9 mIU/mL and 57.6 mIU/mL, respectively. The intra-assay coefficients of variation for FSH were 1.8%, 2.0%, and 1.1% for the mean concentration of 4.0, 9.0, and 50.7 mIU/mL, respectively. The interassay coefficients of variation for FSH were 4.4% and 2.4% for the mean concentration of 15.0 and 41.9 mIU/mL, respectively. The detection limits of the assay were 0.2 mIU/mL for LH, 0.1 mIU/mL for FSH, 8 pg/mL for estradiol, and 0.03 ng/mL for testosterone. Bone age was assessed with the Greulich-Pyle method9). The results are represented as mean±standard deviation in normative data and median (range) in skewed data. Comparison of initial and follow-up height SDS and bone age advancement were analyzed with the Wilcoxon signed-rank test. Statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
Results
1. Etiology and presenting symptoms of GIPP
GIPP was diagnosed at the median age of 2.6 years (range, 0.7-7.9 years), and the median duration of follow-up was 4.6 years (range, 1 month–9.8 years). Among the 7 female patients who presented with vaginal bleeding, 4 had MAS and 3 had FOCs. The diagnosis of MAS was made when the patient showed at least 2 of classic triad including precocious puberty, café-au-lait spot, and polyostotic fibrous dysplasia. Pubic hair had developed in 4 female patients with adrenocortical tumors (adrenocortical carcinoma in 2 and adrenocortical oncocytic adenoma in 2), and in 1 male patient with β-hCG producing germinoma. Three patients presented with breast development: 1 with MAS, 1 with FOCs, and 1 that was idiopathic. One male patient presented with elongated penile length of unknown etiology (Table 1).
2. Laboratory and image findings
Laboratory testing revealed suppressed LH and FSH levels with elevated estradiol in females or testosterone in males (Table 2). In 4 patients with adrenocortical tumors, DHEA-S levels were markedly elevated (median, 936.5 µg/mL; range, 87.2–6530 µg/mL). Peak LH levels were less than 5 mIU/mL, and there were FSH-predominant patterns after exogenous GnRH stimulation (Table 2). GnRH stimulation testing was not done in 3 patients, including 1 with MAS who presented with breast development at the age of 2.5 years, and 2 with adrenocortical carcinomas diagnosed at the ages of 8 months and 2.6 years, respectively.
The median value of difference between bone age and chronological age at the time of diagnosis in the patients with MAS, FOCs, adrenal tumors, and β-hCG producing germinoma was 0.4 years (range, 0–1 years), 0.6 years (range, 0–3 years), 2.8 years (range, 0.4–5.4 years), and 5.1 years, respectively. The radiologic findings in the patients with MAS, adrenocortical carcinoma, and β-hCG producing germinoma are shown in Table 2 and Fig. 1. Two patients (patients 14 and 15) with markedly elevated sex hormone who diagnosed as idiopathic GIPP did not identified any abnormalities in tumor markers and radiologic evaluation.
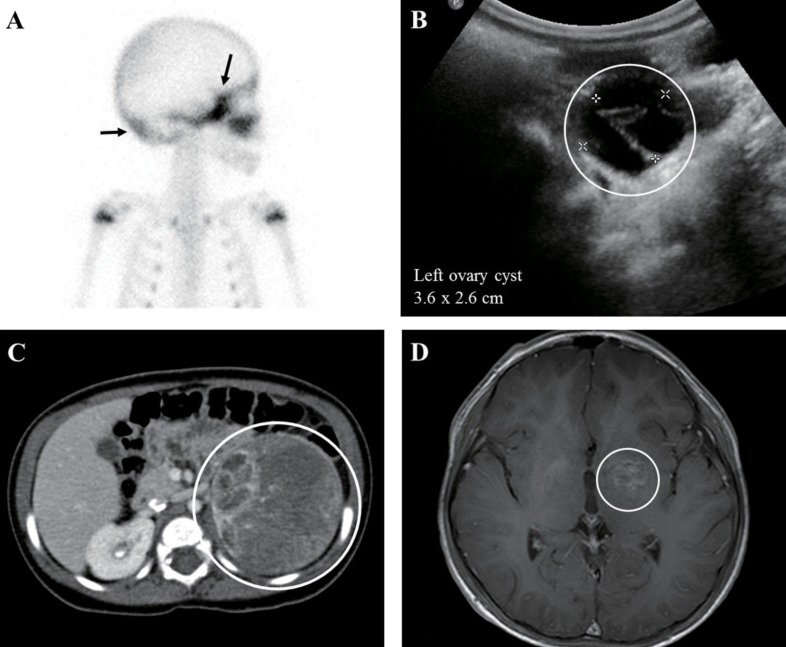
Imaging findings of patients with gonadotropin-independent precocious puberty. (A) Tc-99m 3,3-diphosphono-1,2-propanedicarboxylic acid bone scan (right lateral view) of a patient with McCune-Albright syndrome (MAS) (patient 5) revealed diffuse symmetrical increased uptake along the skull base and occipital bone (arrows), suggesting fibrous dysplasia. (B) 3.6×2.6-cm-sized left ovarian cyst (circle) demonstrated by pelvic ultrasonography in a patient with MAS. (C) Abdominal computed tomography demonstrated an 8.4×6.4-cm left adrenal gland mass with heterogeneous enhancement and calcification (circle) in an 8-month-old girl with premature pubarche (patient 13). (D) Brain magnetic resonance imaging revealed a 2.2×1.9×2.2-cm lobulated mass involving the left basal ganglia (circle) with thickening of the pituitary stalk, indicating germinoma in an 8-year-old boy with premature adrenarche (patient 14).
3. Treatment and outcomes
Five patients with MAS and 3 with FOCs were treated with letrozole. Recurrent vaginal bleeding (median, 0.8 episodes/yr; range, 0.3–1.9 episodes/yr) was observed despite treatment with the aromatase inhibitor. Ovarian cystectomy was not done in any patients in spite of the recurrent vaginal bleeding. Tumor excision was performed for all adrenocortical tumors. The monitoring of tumor recurrence was done by DHEA-S level and imaging study of adrenal glands. Premature pubarche disappeared after tumor excision and there was no recurrence of tumor. The chemotherapy was administered for the β-hCG producing germinoma.
After the diagnosis of GIPP, the patients were evaluated for pubertal stage, growth velocity and bone age every 6 months. The patients who revealed rapid linear growth and advanced bone age were screened for GDPP. During the follow-up period, 6 patients (2 with MAS, 2 with FOCs, and 2 with adrenocortical tumors) transited to GDPP at the median age of 8.0 years (range, 2.8–8.6 years) and median 3.3 years (range, 0.3–5.1 years) after the onset of GIPP. Initial and last height-SDS (0.99±0.84 vs. 1.10±1.10, P=0.44) and bone age advancement (1.49±1.77 years vs. 2.02±1.95 years, P=0.06) were not significantly different (Table 3).
Discussion
In this study, the MAS and FOCs were relatively common cause of GIPP. In these cases, the typical presenting symptom of precocious puberty was vaginal bleeding, which represents withdrawal bleeding following the resolution of estrogen-producing cysts. These patients did not reveal significant bone age advancement at diagnosis. The patients with adrenocortical tumors were presented with virilization and associated with adrenal androgens. There was no tumor recurrence after complete resection.
MAS is a genetic disorder characterized by the triad of café-au-lait spots, polyostotic fibrous dysplasia, and precocious puberty. The manifestations of MAS are due to somatic activating mutation of the GNAS gene encoding the Gsα protein, which is involved in intracellular cAMP production10). GIPP occurs in 50%–90% of female patients with MAS51112). The natural history of precocious puberty in MAS is extremely heterogeneous; some patients experience waxing and waning breast development with or without isolated episodes of vaginal bleeding and minimal bone age advancement, while others experience progressive pubertal maturation and frequent bleeding5). Recurrent exposure to sex hormones accelerates maturation of the epiphyseal plate, resulting in compromised final height, while deformities and fractures of the long bones are caused by polyostotic fibrous dysplasia13).
The long-term management of GIPP in MAS is not established. Girls with infrequent vaginal bleeding can often be observed without treatment. In case of progressive forms of precocious puberty, medications that decrease estrogen biosynthesis or blunt the effects of estrogen should be considered5). In a few studies, letrozole (aromatase inhibitor), tamoxifen (selective estrogen receptor modulator), or fulvestrant (estrogen receptor antagonist) were effective in decreasing the rate of skeletal maturation and vaginal bleeding141516). However, most of these agents have demonstrated inadequate efficacy. In the present study, most patients with MAS or FOCs were treated with letrozole. However, the efficacy was uncertain due to the duration of treatment being short and inconsistent in each patient. None of the subjects' final adult height was measured, therefore, the efficacy of this treatment in GIPP patients needs to be evaluated after long-term follow-up.
Childhood adrenocortical tumors are another entity causing GIPP. Adrenocortical tumors are rare in childhood, but they are the most frequent extracranial tumors that cause GIPP17). The majority of adrenocortical tumors in childhood are functional, and the most common presenting symptom is virilization alone or in combination with other signs of overproduction of adrenal hormones, occurring in 80%–95% of patients181920). In particular, adrenocortical carcinoma should be suspected in girls younger than 6 years old who manifest pubarche with highly elevated DHEA-S levels202122). In the present study, 4 female patients with adrenal tumors demonstrated virilization and markedly elevated serum DHEA-S levels, indicating excessive adrenal androgen production. In our patients, there were no features of Cushing syndrome or mineralocorticoid excess. Age younger than 4 years, virilizing tumor, small tumor size, and localized disease are associated good prognosis in pediatric adrenocortical tumor. The survival rate is approximately 80%–90% in the patients with better prognostic factors2023).
Long-term exposure to sex hormones due to the GnRH-independent process can lead to GDPP, necessitating GnRH agonist treatment. This occurs after initiation of treatment for the underlying disease, particularly in patients with significantly advanced bone age. Once the negative feedback effect of pubertal sex steroid concentrations is removed, the hypothalamic-pituitary-gonadal axis can subsequently be activated. However, the mechanisms are not yet well understood342425). In the present study, transition to GDPP occurred in 6 patients, approximately 3.2 years after the diagnosis of GIPP. This indicates that surveillance for GDPP should be a part of the follow-up in patients with GIPP.
In conclusion, the etiologies of GIPP are heterogeneous, and treatment and prognosis is quite different according to the etiology. It is important to consider the rare etiologies of GIPP and correct the underlying pathology based on the exact diagnosis in patients with GIPP. The medical treatment can be considered although long-term efficacy of these treatments needs to be evaluated. Also, the regular monitoring of GDPP is required during the follow-up of the patients with GIPP.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.