Associations between serum vitamin D levels and precocious puberty in girls
Article information
Abstract
Purpose
Vitamin D deficiency has been linked to chronic diseases, such as diabetes mellitus, obesity and autoimmune disease. However, data on the vitamin D status and its association with precocious puberty in girls are limited. We aimed to investigate the association between serum 25-hydroxyvitamin D (25OHD) and precocious puberty in girls.
Methods
A total of 60 girls with central precocious puberty (CPP) and 30 control girls were enrolled. Anthropometric measurement and serum level of 25OHD were estimated for all subjects.
Results
There was a significant difference in the mean serum 25OHD concentration between the precocious puberty group and the control group (17.1±4.5 ng/mL vs. 21.2±5.0 ng/mL, P<0.05). Forty-two of the 60 girls with CPP (70%) had vitamin D deficiency (defined as serum 25OHD<20 ng/mL) and 18 (30%) had vitamin D insufficiency. Of the 30 girls in the control group, vitamin D deficiency was seen in 13 subjects (43.3%), 15 subjects (50%) had vitamin D insufficiency, and 2 subjects (6.7%) had sufficient serum vitamin D (defined as serum 25OHD>30 ng/mL). Vitamin D deficient girls had a significantly higher odds ratio (OR, 3.05; 95% CI, 1.22-7.57, P=0.021).
Conclusion
These results showed that vitamin D levels may be associated with precocious puberty. Further studies are required to establish the potential effect of vitamin D status on puberty.
Introduction
Vitamin D plays a crucial role in calcium and phosphorus metabolism throughout life. Main action of vitamin D includes intestinal calcium absorption and renal calcium reabsorption1).
Mounting epidemiological studies have reported that vitamin D was associated with risk for many disorders, including certain types of cancer, neurologic disorders, infectious disease, cardiovascular disease, type 2 diabetes mellitus, and autoimmune disorders1,2)
Central precocious puberty (CPP) is characterized by early activation of the hypothalamic-pituitary-gonadal axis before 8 years in girls and 9 years in boys3). Recent studies reported that vitamin D status was associated with the timing of menarche4) and vitamin D modulates reproductive function in women and men5,6). However, there are no reports on the relationship between vitamin D levels and precocious puberty. The aim of this study was to determine the association of vitamin D status with CPP. This study compared the blood levels of vitamin D between girls with precocious puberty and girls with normal pubertal development.
Materials and methods
1. Study population
This cross-sectional study enrolled 60 girls with CPP (children and adolescents). Precocious puberty was defined according to the following criteria: objective breast budding appearing before 8 years of age, advanced bone age (1 year greater than the chronological age), and peak stimulated luteinizing hormone (LH)≥5.0 IU/L on immunoradiometric assay (IRMA) during gonadotropin-releasing hormone (GnRH) stimulation7). To avoid seasonal variations, the study was undertaken at Ajou University Hospital, Suwon, Korea (latitude 37°), between April 2012 and June 2012. Exclusion criteria were precocious puberty with an identified etiology, such as brain tumor or cranial irradiation. Plasma 17-hydroxyprogesterone was measured to exclude abnormal androgen secretion. Serum thyroid function was measured to exclude hypothyroidism. Ovarian disorder was ruled out on the basis of pelvic ultrasound. We recruited 30 Korean girls with normal growth and development who had been followed at our pediatric growth clinic. Subjects taking medications known to affect the reproductive axis were also excluded and none of the study subjects had used hormonal medications before the study.
The study protocol was approved by the Ajou University Institutional Review Board and was in concordance with the Helsinki declaration. Written informed consent was obtained from the legal guardian of all the participants and the patient's consent was also obtained.
2. Study design
The serum 25-hydroxyvitamin D (25OHD) level of all the subjects was measured by radioimmunoassay. The GnRH stimulation test was performed at day time. Basal serum samples were obtained prior to GnRH injection (GnRH 100 µg/m2 IV), and post stimulation samples were acquired 30, 45, 60, and 90 minutes after the injection for measurement of LH, FSH, and Estradiol (E2) levels. The subjects' height, weight, pubertal status, thyroid function, and bone age were collected from the review of clinic charts and electronic medical records. Pubertal status (Tanner stage for breast development) was assessed and documented by one pediatric endocrinologist. Patients were categorized as being in the pubertal stage once they have reached Tanner 2-5) Bone age was measured using the method described by Greulich and Pyle8). BMI was calculated and the BMI standard deviation scores (SDSs) were derived using the 2007 Korean National Growth Charts9).
3. Laboratory Measurements
The detection limits for serum LH and FSH levels measured by IRMA (BioSource, Nivelles, Belgium) were 0.2 IU/L and 0.1 IU/L, intra-assay coefficient of variation (CV) ranging from 1.4%-3.9% and 1.1%-2.0%, and inter-assay CV ranging from 3.4%-8.0% and 2.4%-4.4%, respectively. E2 levels were determined via radioimmunoassay with analytical sensitivity of 5 pg/mL, intra-assay CV ranging from 4.0%-7.0%, and inter-assay CV ranging from 4.2%-8.1% (radioimmunoassay; Coat-A-Coung, Diagnostic Products, Los Angeles, CA, USA). Serum 25OHD concentration, as a marker of vitamin D status, was measured with a radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA) using a gamma counter (1470Wizard, PerkinElmer, Turku, Finland). The following are the definitions of the various vitamin D states used in this study: sufficient vitamin D state, 25OHD of at least 75 nmol/L (30 ng/mL); vitamin D insufficiency, less than 75 nmol/L (30 ng/mL); and vitamin D deficiency, less than 50 nmol/L (20 ng/mL)10).
4. Statistical analysis
The clinical and laboratory parameters from the CPP and control groups were compared using the Student t-test. Subjects were divided into three subgroups according to their vitamin D status. The odds ratios (ORs) of precocious puberty depending on vitamin D levels were calculated by binary logistic regression. Statistical analysis was performed using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). Statistical significance was defined as P<0.05. Results are described as mean±standard deviation (SD) unless otherwise stated.
Results
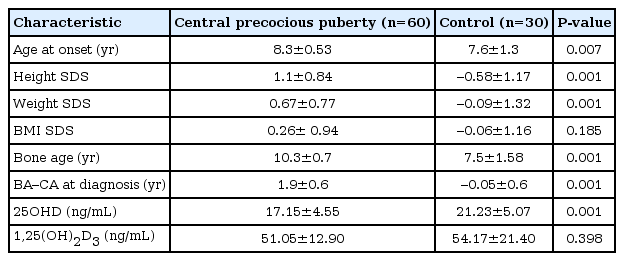
A total of 90 subjects participated in this study (CPP, 60; control subjects, 30). The mean age of the subjects was 8.09±0.94 years. The mean 25OHD level was 18.5±5.0 ng/mL. Most of the subjects (97.7%) did not reach sufficient status. Assessment of pubertal status by the Tanner method in the CPP group showed that 35 girls were in Tanner stage 2, 20 girls in Tanner stage 3, and 5 girls in Tanner stage 4. Clinical and laboratory characteristics of the CPP and control group are shown in Table 1. Mean 25OHD levels were significantly lower in children with precocious puberty (17.1±4.5 ng/mL compared to 21.2±5.0 ng/mL). In addition, children with CPP showed a significantly higher height SDS and weight SDS compared with the control group. There was no significant difference in BMI SDS between the CPP and control group.
Subjects in the CPP group were further divided into two subgroups on the basis of the serum 25OHD values to compare the clinical characteristics and hormone values. The proportion of girls with advanced pubertal stage (Tanner 3 and 4) was higher in the vitamin D deficiency subgroup than in the vitamin D insufficiency subgroup, although there was no statistical significance (P=0.052). No significant difference was seen in basal LH, peak LH, basal FSH, peak FSH, basal E2, peak E2 and bone age between the two subgroups (Table 2).
Table 3 compared the rates of CPP against the vitamin D status. Forty-two of the 60 girls with CPP (70%) had vitamin D deficiency (defined as serum 25OHD<20 ng/mL) and 18 (30%) had vitamin D insufficiency. Of the 30 subjects in the control group, vitamin D deficiency was seen in 13 subjects (43.3%), 15 subjects (50%) had vitamin D insufficiency, and 2 subjects (6.7%) had sufficient vitamin D (defined as serum 25OHD>30 ng/mL). Vitamin D deficient girls had a significantly higher odds ratio (OR, 3.05; 95% confidence interval [CI], 1.22-7.57; P=0.021). Also, the ORs of precocious puberty increased 1.19 fold in girls with decreased vitamin D levels in logistic regression analysis after adjusting weight SDS, BMI SDS, age (Table 4).

The multivariate analysis by logistic regression test to analyze the relationship between precocious puberty and vitamin D
Pearson correlation coefficient analyses revealed no relationship between serum 25OHD and weight SDS (r=-0.153, P=0.150), BMI SDS (r=-0.086, P=0.418), peak LH (r=-0.046, P=0.729), and peak E2 (r=-0.099, P=0.450). However, a significant inverse correlation was found between 25OHD and the difference of bone age and chronological age (r=-0.273, P=0.009).
Discussion
Our study showed that the level of 25OHD was significantly lower in girls with CPP compared to those in normal control subjects. Among subjects in the precocious puberty group, 70% had deficiency in vitamin D, compared to 43.3% from the group with normal pubertal development. Within the CPP group, advanced Tanner stage (Tanner stage 3 and 4) was more frequently observed in the vitamin D deficiency subgroup. Girls with CPP had higher risk of vitamin D deficiency (OR, 3.05; 95% CI, 1.22-7.57). This is the first report examining the association between serum 25OHD levels and precocious puberty in girls.
Recently, many reports showed an increase in the incidence of CPP and a decline in age at pubertal onset in girls3,11). Although a lot of studies have reported on precocious puberty, the etiology underlying the early activation of the hypothalamic-pituitary-gonadal axis is still unclear. Genetic factors are the major contributors in the timing of puberty12,13). However, the observed secular trend in pubertal onset with a steady decline of the age at onset over the past decades clearly suggests that an environmental factor may be influencing the current change in pubertal progression14,15). In the last few years, the prevalence of vitamin D deficiency has been increasing in many parts of the world16). Vitamin D plays a key role in bone metabolism, being important for the maintenance of calcium homeostasis by intestinal and renal calcium absorption2). The vast majority of the effects of vitamin D are mediated by the vitamin D receptor (VDR), which is the only protein that binds 1,25(OH)2D317). The VDR is expressed in almost all body cells, such as immune, vascular cells as well as ovary and human pituitary gland18,19). This has led to extensive research on vitamin D as a potential influencing factor in the pathogenesis of a number of nonskeletal diseases, including infectious and autoimmune diseases, obesity, cancer and fertility20).
There are few studies looking at the association of vitamin D status and sexual maturation and female reproduction. The role of vitamin D deficiency on precocious puberty is not clear. In previous animal studies, vitamin D deficiency has lead to reduction in overall fertility of female rats compared to vitamin D-replete ones by direct regulation of aromatase gene expression18,21). In humans, the VDR gene polymorphism at the ApaI site is significantly associated with the earlier age at menarche22). In one study, age at menarche was earlier in girls living at higher geographic latitude than girls living towards the equator23). Villamor et al.4) followed a cohort of 242 healthy girls (age, 5-12 years) for a median of 30 months and found that vitamin D deficient girls have an earlier initiation of menstruation than girls with sufficient vitamin D. They explained that vitamin D deficiency was associated with obesity, so vitamin D status could indirectly affect the age at menarche by its effect on obesity. In our study, girls with CPP were also heavier than the normal control subjects, although the BMI SDS was not significantly differ between the two groups. The other possible mechanism was that vitamin D had inverse correlation with insulin-like growth factor-1 (IGF-1)24). IGF-1 modulates the onset of puberty and pubertal progression by stimulating the GnRH25). So, it is conceivable that vitamin D-mediated effects may influence IGF-1 levels and pubertal onset through an effect on gonadotropin and sex hormone.
This study has a few limitations stemming from relatively small sample size. The most subjects had vitamin D deficiency and insufficiency except two control girls. Also, we were unable to assess other hormones that affect the pubertal onset, such as IGF-1, as a result, causality cannot be proven.
In conclusion, our study indicated that vitamin D deficiency was more common in girls with CPP than girls with normal patterns of sexual maturation. Although the mechanism of vitamin D deficiency's effect on pubertal progression is unclear, we suggest that vitamin D may influence sexual maturation in girls. Further studies including more vitamin D sufficient subjects are required to establish the potential effect of vitamin D status on the development of puberty.
Notes
No potential conflict of interest relevant to this article was reported.