A novel variant of THRβ and its 4-year clinical course in a Korean boy with resistance to thyroid hormone
Article information
Abstract
Thyroid hormone resistance (RTH) is characterized by a decreased sensitivity of target tissues to thyroid hormones due to a defect in the THRα- and THRβ-encoded thyroid hormone receptors (THRs). The clinical manifestations range from no symptoms to simple goiter and hypo- or hyperthyroidism, depending on the receptor subtype distribution in the tissues. Here, we report the case of a thyroid hormone-resistant 12-month-old boy carrying a novel THRβ variant who was initially diagnosed with congenital hypothyroidism. An extensive evaluation revealed increased free T4 level and inappropriately increased thyroid-stimulating hormone (TSH) level; a normal lipid profile, sex hormone-binding globulin, and free alpha subunit of TSH; exaggerated TSH response to THR; and no radiological evidence of pituitary adenoma. A targeted next-generation sequencing panel identified a heterozygote c.993T>G (p.Asn331Lys) mutation in the THRβ gene. During the first year of life, a higher dose of levothyroxine was administered to the patient due to uncompensated RTH. Levothyroxine treatment was continued after 3 years to maintain TSH level <5 mIU/mL, but the observed weight gain was poor, height increase was insufficient, and bone development was delayed. However, neither hyperactivity nor developmental delay was observed. Patients with RTH exhibit various clinical features. Due to its heterogeneous nature, genetic test for accurate diagnosis is important to provide proper management.
Highlights
· Thyroid hormone resistance (RTH) is a syndrome characterized by reduced target organ sensitivity to thyroid hormone,which present a various clinical phenotypes, often leading to misdiagnosis. We report the 4-year clinical course of a boy who diagnosed with RTH due to novel variant mutation in THRβ.
Introduction
Thyroid hormone (TH) resistance (RTH) is characterized by reduced target tissue sensitivity to TH, elevated serum TH level, and failure to suppress pituitary thyroid-stimulating hormone (TSH) secretion. RTH was first described in 1967 [1], and its prevalence is 1:40 ,000 [2]. RTH is caused by a defect in THRα- and THRβ-encoded thyroid hormone receptors (THRs). Approximately 90% of RTH patients carry a typically autosomal-dominant THRβ mutation; at least 236 mutations have been identified in 805 families [3]. THR isoforms are distributed in various tissues and present varying degrees of hormonal resistance depending on the relative levels of gene expression, resulting in varying symptoms. A patient may show signs and symptoms of hypothyroidism in one tissue and suggestive thyrotoxicosis in others. Some patients might be asymptomatic and in a euthyroid state [3,4], suggesting that RTH can be easily misdiagnosed based on symptoms or thyroid function tests. Genetic tests are diagnostic tools that can reduce unnecessary drug administration and promote individualized treatment. Here, we report the 4-year clinical course in a Korean boy with RTH with a novel THRβ variant, c.993T>G (p.Asn331Lys).
Case report
A 12-month-old boy, referred to the Department of Pediatric Endocrinology for abnormal thyroid function test results, presented with hyperthyroxinemia and an inappropriately increased TSH level. He was a first-born, delivered by cesarean section at 28 weeks 2 days of gestation because of premature membrane rupture, and had no family history of goiter or autoimmune thyroid disease (AITD). His birth weight, length, and head circumference were 1,100 g (-0.08 standard deviation score [SDS]), 35 cm (-0.76 SDS), and 27 cm (0.78 SDS), respectively, and his 1- and 5-min Apgar scores were 7 and 8, respectively.
After birth, prophylactic surfactant replacement therapy and an invasive ventilator were initiated. A physical examination at 6 days after birth did not indicate goiter; however, a routine venipuncture thyroid function test revealed a TSH level of 16.67 μIU/mL (reference range, 0.35–4.94 μIU/mL) and a free T4 (fT4) level of 2.47 ng/dL (reference range, 0.7–1.48 ng/dL). The pulse rate was 154–166 per minute (reference rate, 140–184 per minute). Total bilirubin and direct bilirubin were 7.4 mg/dL and 0.78 mg/dL, respectively. Although venous TSH concentration was <20 μIU/mL, levothyroxine (10 μg/kg/day) was initiated. At 49 days after birth, TSH and fT4 levels were 75.9 μIU/mL and 1.2 ng/dL, respectively, and levothyroxine dose was increased to 30 μg/kg/day. At 12 months, an elevated fT4 (3.22 ng/dL) and an inappropriately high TSH level (2.47 μIU/mL) continued, and the patient was referred to the Department of Pediatric Endocrinology. He had no history of heat intolerance, excessive sweating, weight loss, constipation, or developmental delay on admission. His age-corrected height was 73 cm (-0.12 SDS), weight 7.42 kg (-1.92 SDS), blood pressure 90/60 mmHg (reference range, 80–100/55–65 mmHg), pulse rate 84 beats/min (reference range, 80–120 beats/min), and body temperature was 36.7℃, which were all within the normal ranges. On physical examination, goiter, thrill over the thyroid gland, and exophthalmos were not observed. His developmental quotient by Korean infant and toddler development-screening tests was within the normal range for his corrected age. His thyroid function test revealed serum fT4, T3, and TSH levels of 2.89 ng/dL, 1.9 ng/mL, and 5.33 μIU/mL, respectively. Normal levels of total cholesterol (137 mg/dL; reference range, <200 mg/dL), triglycerides (63.8 mg/dL; reference range, <130 mg/dL), highdensity lipoprotein cholesterol (46.9 mg/dL; reference range, >40 mg/dL), and low-density lipoprotein cholesterol (77.3 mg/dL; reference range, <100 mg/dL) were noted. Hemoglobin concentration was 13.9 g/dL (reference range, 11.3–14.1 g/dL). The results of antithyroglobulin antibody and TSH receptor antibody tests were negative, whereas that of antimicrosomal antibody was positive (12.83 IU/mL; reference range, <12 IU/mL). Free alpha subunit (0.3; reference range, <0.7 ng/mL) and sex hormone-binding globulin (SHBG; 123.8 nmol/L; reference range, 41.5–150 nmol/L), which are associated with TSHsecreting tumor, were not elevated.
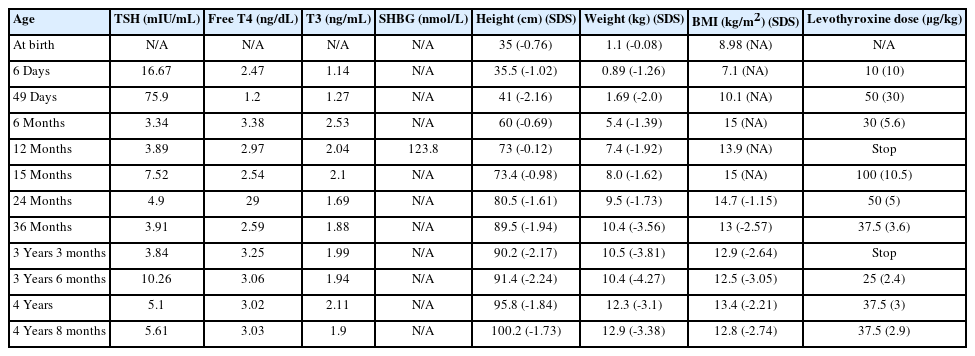
The thyrotropin-releasing hormone stimulation test showed an exaggerated TSH response (TSH at baseline, 7.02 μIU/mL; at 30 minutes, 88.55 μIU/mL; at 60 minutes, 73.4 μIU/mL; at 90 minutes, 79.27 μIU/mL; at 120 minutes, 64.41 μIU/mL). Magnetic resonance brain imaging revealed no evidence of pituitary adenoma; however, Rathke cleft cyst was found (Fig. 1). Thyroid ultrasonography showed normal size, contour, parenchymal echogenicity, and vascularity without nodules. The 99m Technetium thyroid scan revealed increased uptake in both normal-sized thyroid lobes.
Sella magnetic resonance imaging scan of the patient at 12 months of age. Sagittal T1-weighted image was normal except for Rathke cleft cyst (arrow).
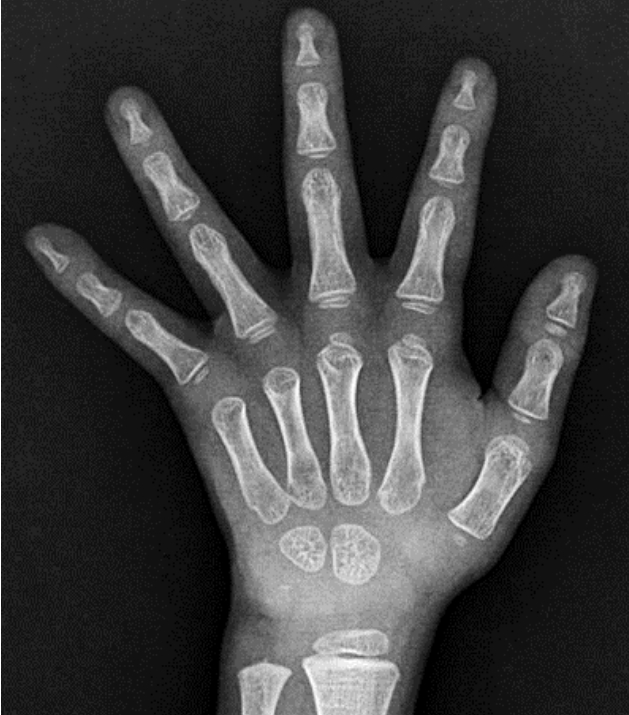
Owing to the possibility of RTH, a targeted next-generation sequencing panel test associated with hypothyroidism that included THRα, THRβ, SLC16A2, DUOX2, DUOXA2, FOXE1, GNAS, HESX1, IYD, LHX3, NKX2-1, NKX2-5, PAX8, POU1F1, PROP1, SLC26A4, SLC5A5, TG, TPO, TRH, TRHR, TSHB, and TSHR was conducted after obtaining informed consent from his parents. Genomic DNA was extracted from the peripheral blood of the patient. The test revealed a preheterozygote c.993T>G (p.Asn331Lys) mutation in THRβ. (Figs. 2, 3) An in silico analysis (MutationTaster, PolyPhen-2, and SIFT) predicted this mutation to be deleterious (PP3). The variant was novel (http://www.hgmd.cf.ac.uk, access date: November 15, 2021.) and located at a mutational "hot spot" (PM1) that codes for the T3-binding domain [5]. According to the data from the 1000 Genomes Browser (2,504 individuals, http://phase3browser.1000genomes.org) and ExAC (60,706 individuals, http://exac.broadinstitute.org/) databases and gnomAD v2.1.1 (https://gnomad.broadinstitute.org), this variant was absent in control individuals (PM2). Moreover, a missense pathogenic mutation including c.993T>G (p.Asn331Lys) has been reported at the same site (PM5) [6]. Therefore, the variant was classified as likely pathogenic according to the ACMG/AMP guidelines (≥3 moderate and 1 supporting). The patient had no siblings, and the thyroid function of his parents was normal; for economic reasons, the parents did not agree to undergo genetic tests on themselves.
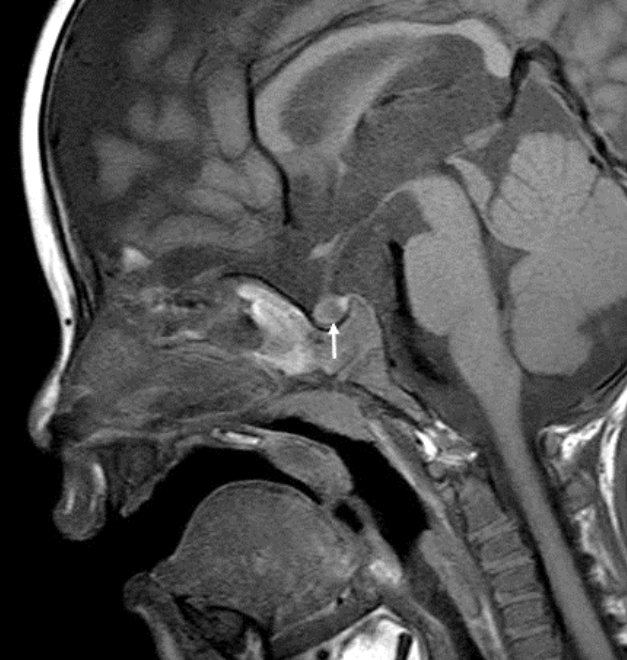
Bone age radiograph at the chronological age of 4 years 8 months. The bone age was 3 years using the Greulich and Pyle method.
(A) Pedigree of the patient. (B) Exome sequencing for genetic analysis. Heterozygous missense variant c.993T>G (p.Asn331Lys) was found in THRB.
During the administration of levothyroxine, sinus tachycardia (pulse rate 88–131 beats/min) was observed in the outpatient clinic; however, heat intolerance or hyperactivity was not. Finally, the boy was diagnosed with RTH and transferred to another hospital, closer to his hometown, where the physician restarted levothyroxine to maintain a TSH level < 5 μIU/mL. The dose was gradually reduced, and the drug was discontinued at 3 years. However, after 3 months of levothyroxine discontinuation, TSH level had increased to 10.2 μIU/mL without goiter, and 2–3 ug/kg/day levothyroxine was readministered. At 4 years 8 months, his TH level was elevated with unsuppressed TSH level (TSH, 5.61 μIU/mL; fT4, 3.03 ng/dL; T3, 1.9 ng/mL) (Table 1). At that time, the patient showed insufficient growth and weight gain. His height was 100.2 cm (-1.73 SDS), weight was 12.9 kg (-3.38 SDS), and body mass index (BMI) was 12.8 kg/m2 (-2.73 SDS); however, his development was normal. His bone development was delayed with respect to his chronological age (Fig. 4).
Discussion
RTH exhibits various clinical manifestations and is often misdiagnosed as hypo- or hyperthyroidism, resulting in ineffective drug or surgical treatment. Ablative treatment or surgical resection under the diagnosis of hyperthyroidism or goiter requires TH administration, often in supraphysiological doses [7]. Differential diagnoses include TSH-secreting pituitary adenoma, familial dysalbuminemic hyperthyroxinemia, and assay interference. RTH should be suspected if the following conditions are present: (1) absence of elevated α pituitary glycoprotein-subunit serum concentration, (2) normal or increased TSH response after TRH administration, (3) family history of RTH, (4) absence of elevated serum SHBG concentration, reflecting a euthyroid state, (5) suppression of serum TSH level with supraphysiological doses of L-T3, and (6) identification of THRα and THRβ mutations by genetic testing [8].
The molecular structures and sequences of THRα and THRβ are similar; 4 isomers are denoted as THR-α1, -α2, -β1, and -β2 [9]. THRα1 is expressed primarily in the heart, bone, and brain, whereas THRα2 is widely distributed but is unable to bind to hormones. THRβ1 is predominantly expressed in the liver and kidney, and THRβ2 is highly expressed in the pituitary gland and hypothalamus [10,11]. Therefore, symptoms of TH deficiency and excess could coexist in different tissues of the same patient. For example, growth retardation and learning difficulty (suggestive of hypothyroidism) may coexist with weight loss, osteoporosis, and tachycardia (typical of thyrotoxicosis) [4]. Tachycardia may be explained by high THRα1 concentration in heart cells [7].
Most THRβ mutations are concentrated in 3 "hot spots" between exons 7 and 10 (234–282, 310–353, 429–461) [12]; in the present case, c.993T>G (p.Asn331Lys) was identified. To our knowledge, only 3 patients (patients 1, 2, and 3) with RTH who had mutation at codon 331 in exon 9 have been previously reported (Table 2) [5,6,13]. Patient 4 was the patient of the present report. Patients 1 and 2 inherited THRβ mutations from their mothers, and the other exhibited de novo mutation. On physical examination, goiter was found in 2 of the 3 patients. Tachycardia was investigated in patients 1 and 4, and heart rate was within the normal range in both. Anthropometric parameters were measured for patients 1, 3, and 4. Height and BMI standard deviations varied from 0.9 to -1.75 and 0 to -2.73, respectively. Measurements in patients 1 and 4 indicated delayed bone age compared with chronologic age. TSH, fT4, and T3 levels were 2.2–15.2 μIU/mL, 2.2–2.89 ng/dL, and 1.9–8 ng/mL, respectively. Antimicrosomal antibodies were found in the 3 tested patients. Antithyroglobulin antibodies were found in 1 of 2 patients who underwent the examination. Long-term outcome was reported in patient 1, who experienced late puberty, no learning difficulty, and a normal adult life with 0.02 mg/kg/day triiodothyroacetic acid treatment.

Clinical and biochemical features of patients with thyroid hormone resistance who had mutation at codon 331 in exon 9
In most patients with RTH, high level of circulating TH might compensate for partial tissue resistance. Therefore, RTH treatment should be tailored to correct the coexistence of hypo- and hyperthyroidism in various tissues. Selective beta-blockers can be considered for patients with tachycardia or tremor. Triiodothyronine (L-T3) treatment may benefit goiter, hyperactivity, and mental clouding [3]. Administering supraphysiologic L-T3 (250 μg) every other day effectively reduced goiter size in large symptomatic goiter cases [14]. Levothyroxine treatment is indicated when RTH coexists with thyroid dysgenesis, such as in ectopic thyroid glands or hypothyroidism due to autoimmune thyroiditis [15]. Supraphysiologic levothyroxine is often needed to maintain serum TSH at the lowest tolerable level [3]. Additionally, these symptoms and basal metabolic rate, nitrogen balance, and serum SHBG should be monitored at each dose escalation [16].
Indeed, RTH patients had a higher frequency of AITD, in which elevated TSH stimulates intrathyroidal lymphocytes, which results in the production of proinflammatory cytokines and the destruction of thyrocytes [17]. Meanwhile, patients with RTH due to THRβ mutation have an approximately 2-fold higher risk of AITD compared to unaffected relatives, but the prevalence of thyroid autoantibodies with aging is not affected by genotype [18]. In the present case, antimicrosomal antibody was positive at 12 months of age, but it was just above the normal range. Normal radiographic findings were observed on thyroid scan and ultrasound. Serological evidence of thyroid autoimmunity including antithyroglobulin antibody, TSH receptor antibody, and antimicrosomal antibody were not observed around age 3 or 4. For proper management of this patient, further regular evaluation for thyroid autoimmunity is needed.
Treatment guidelines for neonatal patients with RTH and congenital hypothyroidism are lacking. As previously mentioned, L-T3 treatment could be an option for patients with RTH; however, L-T3 has not been reported administered for congenital hypothyroidism [19]. Therefore, a clinician can consider levothyroxine treatment. The goal of treatment with supraphysiologic levothyroxine doses is to return serum TSH level to near normal while monitoring cognitive development, bone maturation, and growth [3]. To maintain TSH below 5 μIU/mL, levothyroxine (up to 350 μg/day) was administered to a young female patient presenting RTH combined with ectopic thyroid glands [20]; however, prolonged treatment with levothyroxine after age 3 resulted in poor weight gain.
In conclusion, RTH does not exhibit specific clinical features but may be associated with tachycardia, goiter, growth retardation, or other symptoms. Due to its heterogeneous nature, each patient should be individually evaluated and treated. If RTH is suspected, clinicians should consider either a thyroid scan or ultrasound to check for thyroid dysgenesis or laboratory examinations to determine hypothyroidism associated with autoimmune thyroiditis. Long-term follow-up data and a detailed diagnosis and treatment strategy for RTH are necessary.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: JM, SK; Data curation: SM, SP; Formal analysis: SK, JM; Methodology: SP; Project administration: HK, SK; Visualization: SK, JM; Writing - original draft: SK; Writing - review & editing: HK, SK