New-onset type 1 diabetes mellitus in the Paediatric Emergency Department: impact of the COVID-19 pandemic
Article information
Abstract
Purpose
On the 14th of March 2020, the Spanish government decreed a state of alarm due to the coronavirus disease 2019 (COVID-19) pandemic, directly affecting healthcare. This situation led to delayed diagnosis of several serious diseases, and its impact on many diseases such as the onset of type 1 diabetes mellitus (T1DM) remains unknown. The aim of this study is to determine the impact of the COVID-19 pandemic on the onset of T1DM in children.
Methods
A descriptive-observational study was performed using data from children younger than 18 years (n=115) admitted with diagnosis of T1DM. We compared the 8 months from May–December 2020 to the same timeframe in 2019.
Results
Our data show an increase of newly attended cases of T1DM in 2020, due to referral of Catalan children with onset of diabetes to our centre. Moreover, fewer patients presented with simple hyperglycaemia at the onset of the COVID-19 period. Delay in consulting the hospital, decreased access to the healthcare system, and avoidance of hospitals to minimize exposure to COVID-19 could have contributed to this finding. There were no differences in the number of days of hospitalization (including days in the paediatric intensive care uniy) between the years.
Conclusions
The effects of the lockdown during the COVID-19 pandemic not only delayed the diagnosis of diabetes, but also its allowed time for its severity to increase. Future studies should focus on the influence of new variants of COVID-19 on the onset of T1DM during the postvaccination period.
Highlights
· This is the first review of the impact of the COVID-19 pandemic in new-onset type 1 diabetes mellitus in a Pediatric Hospital in Spain
· Is interesting to demostrate that our hospital became the reference centre for acute and severe illness in our community.
· The COVID-19 pandemic did not altere the management of new-onset type 1 diabetes mellitus in our pediatric emergency deparment.
Introduction
On 11 March 2020, the World Health Organization announced a pandemic caused by a new coronavirus disease (COVID-19) causing a global health crisis [1]. In order to manage the outbreak and reorganize the public health system, the Spanish government decreed a state of alarm (from 14 March to 21 June 2020). Lockdown restrictions compromised access to medical care, and patients with mild symptoms were recommended to stay at home. As a consequence, visits to Paediatric Emergency Departments decreased by 84% [2]. This situation could have delayed new diagnoses of chronic diseases as type 1 diabetes mellitus (T1DM) and its treatments, allowing rapid progression of the disease and its complications [3].
Diabetes mellitus (DM) is composed of a group of metabolic disorders characterised by chronic hyperglycaemia. In paediatrics, T1DM (autoimmune origin) is the most frequent, and there is great variability in the initial clinical presentation. The classic clinical symptoms include polyuria with nocturia, polydipsia, and weight loss for 2 to 6 weeks. Children younger than 2 years tend to exhibit more rapid manifestation and are at greater risk of diabetic ketoacidosis (DKA). The biochemical criteria for diagnosis of DKA as defined by the International Society for Paediatric and Adolescent Diabetes (ISPAD) are hyperglycaemia (blood glucose >200 mg/dL), venous pH < 7.3 or serum bicarbonate <15 mmol/L, ketonemia (blood B-hydroxybutyrate ≥ 3mmol/L), or moderate/severe ketonuria [4]. Close monitoring of children allows early diagnosis and limits progression to DKA.
The aim of this study is to investigate the impact of COVID-19 on new-onset of T1DM, ketoacidosis presentation, and its complications in a paediatric Spanish population and to compare the findings to those of the same period in 2019.
Materials and methods
A descriptive-observational study was performed in a highly specialized children's hospital in Barcelona (Hospital Sant Joan de Déu). We collected data from children younger than 18 years (simple size n=115) admitted with new diagnosis of T1DM. We compared the 8 months of data from the COVID-19 pandemic, May–December 2020, to the same timeframe in 2019. Factors of sex, age, source, ketoacidosis presentation as defined by the ISPAD guidelines [4], disease severity (pH <7.00), admission to the paediatric intensive care unit (PICU), and length of stay were included.
Statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA), specifically Pearson chisquare test (P<0.05 was statistically significant).
This study was approved by the Hospital Sant Joan de Déu Ethics Committee.
Results
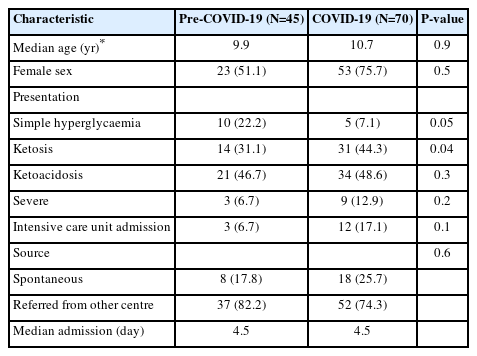
We included 45 patients (39.1%) in the first group (prepandemic, May–December 2019) and 70 patients (60.9%) in the second group (COVID-19 pandemic). There was an increase in incidence of 55% in the second period. The median age was 9.9 years in the first period and 10.7 years in the second. In the first group, 51.1% of the patients were female, while the second group consisted of 75.7% females. In the first group, 17.8% of the patients were admitted to the hospital of their own volition, while 25.7% of the patients in the second group admitted themselves. Regarding presentation, a statistical tendency was observed between the 2 groups, with less frequent simple hyperglycaemia in the second period (22.2% vs. 7.1%) (Fig. 1). There was no statistically significant difference between the 2 groups in days of admission or in patients admitted to the intensive care unit. In Table 1, characteristics of the 2 groups are presented.

Comparison of forms of presentation before and during the coronavirus disease 2019 (COVID-19) pandemic.
Discussion
Our data reveal more cases of newly diagnosed diabetes during the COVID-19 pandemic compared to the same time period in the previous year. Other studies also describe larger numbers of cases of new diagnoses, suggesting that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus exposure could contribute to this increase. However, there is no definitive correlation between the virus and the new cases [5-7]. Moreover, in another study, fewer cases were described in the first wave in 2020 (March–April), while more cases were reported during the entire year in comparison with those in 2019 [8]. A progressive attenuation of control measures could have contributed to an increase of the infection rate while acting as a trigger for the development of T1DM on genetically susceptible children. The SARS-CoV-2 virus was postulated to bind to the angiotensin-converting enzyme 2 receptor in pancreatic islets, eventually causing cell damage, insulin deficiency, and increasing risk of DKA [9,10]. Nevertheless, in our study, only 3% of patients tested positive for SARS-CoV-2 at diagnosis. However, serology results were not reported. Our findings are consistent with studies that showed a low prevalence of COVID-19 among children at T1DM diagnosis [11].
Our hospital turned into a paediatric reference centre for the entire region of Catalonia (Spain) during the lockdown period. As a consequence, almost all Catalan children with the onset of diabetes were referred to our centre during the COVID-19 period, possibly accounting for the increase of new cases of T1DM. However, other studies observed fewer new diabetes cases during the COVID-19 period [12-14]. The reduction of interpersonal interactions during school closures, quarantine, and lack of exposure to seasonal viruses (which is a known precipitating factor for T1DM) may have contributed to this observation.
Moreover, fewer patients presented with simple hyperglycaemia at the onset of the COVID-19 period. According to other studies, more patients presented with DKA at initial diagnosis during the first months of the pandemic [5-7,11,13,15-17]. We did not find differences in the severity of the DKA in our results, in agreement with other studies [10,13]. Nevertheless, other studies noted that patients more frequently presented with more severe forms of DKA during the lockdown [5,6,12,15,16,18,19]. One of the main hypotheses for the increased severity in the onset of T1DM is the delay in consulting the hospital. Decreased access to the healthcare system and the reduction of resources for nonemergency or nonrelated COVID-19 consultations may have contributed to the increased severity. This is highlighted in our cohort with the increase in consultations by patient initiative, rather than referrals from the paediatrician. On the other hand, the population tended to avoid hospitals to minimize exposure to SARS-COV2 [6,11,20,21]. In other studies, more severe forms of DKA and more frequent admissions to the PICU occurred in the first wave, while the severity of newonset diabetes in the second wave was comparable to that of the period before the COVID-19 pandemic [8].
In our study, the number of days of hospitalization (including days in the PICU) was similar in the 2 periods [10]. Other studies found no differences in acute complications and mortality during these 2 periods [8,12]. This could suggest that the differences before and during the COVID-19 pandemic were in presentation. Conversely, once the patient was stabilized and the treatment established, the disease course was similar in the 2 periods.
This study is limited by the retrospective data and by lack of accessibility to hospitals during the COVID-19 pandemic. The decrease in PICU visits also could have misrepresented the data.
In conclusion, the effects of the lockdown during the COVID-19 pandemic affected not only the delay of new diabetes diagnoses, but also the severity of disease.
The retrospective data collection is the main limitation of the study. The lack of serology tests in our patients prevented identification of the possible role of SARS-COV-2 in the induction of T1DM. Moreover, it would have been interesting to analyse the data from the first wave and the second wave separately. Future studies should focus on the influence of new variants of COVID-19 on the onset of T1DM during the postvaccination period.
Among the strengths of the research, this is the first study in Spain to investigate the incidence, the form of presentation, and the severity of newly diagnosed paediatric T1DM cases during the first year of the COVID-19 pandemic and to compare it to the same period in 2019. Our data may contribute to understanding the COVID-19 pandemic impact in new T1DM, as well as to devise strategies to enhance the prognosis of such patients. Early diagnosis of new-onset diabetes is essential to prevent future complications and to improve the quality of life of patients and their families.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: RGR, LBR, NSM; Data curation: NSM; Formal analysis: NSM, VAC, VTSM; Methodology: RGR, LBR, NSM, VAC; Writing - original draft: RGR, LBR, NSM, VAC; Writing - review & editing: RGR, LBR, NSM, VAC, CL