 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(3); 2023 > Article |
|
Abstract
Purpose
Type 1 diabetes mellitus, which is the most common type of diabetes among children, is not curable but can be managed well without a negative effect on quality of life. One of the treatments of type 1 diabetes mellitus is carbohydrate counting. This systematic review and meta-analysis sought to evaluate the efficacy of carbohydrate counting with regard to hemoglobin A1c (HbA1c) reduction in children with type 1 diabetes mellitus.
Methods
Nine studies were assessed, with the primary outcome being glycemic control (HbA1c changes). We searched the following electronic databases: ProQuest, PubMed, Scopus, and ScienceDirect. The quality of studies included was assessed using the risk of bias for randomized control trials and the JBI Critical Appraisal Checklist for observational and cross-sectional studies. Quantitative analyses were made and extrapolated into a forest plot.
Results
A total of 1,693 articles were identified. Four reviewers independently screened titles and abstracts. Of the 36 articles screened, 34 articles were found to be eligible. Of these, 25 studies were excluded because of unsuitable outcomes and study designs. Nine articles were included in the final analysis. Meta-analysis showed that there was a reduction in HbA1c in the carbohydrate counting group as compared to the control group. The cumulative effect of carbohydrate counting on HbA1c was a mean difference of -0.55 (95% confidence interval, -0.81 to -0.28, P<0.001). All of the studies exhibited similar results with the mean difference reduction favoring the interventional group. However, the heterogeneity analysis revealed an I2 value of 88%, implying high heterogeneity in the meta-analysis.
· Carbohydrate counting is one of the treatments for type 1 diabetes mellitus (T1DM). It shows a significant effect in reducing glycated hemoglobin in the children population. The use of carbohydrate counting can be enforced in T1DM management.
Diabetes mellitus can occur in children and adults. It is a metabolic syndrome in which the body undergoes inappropriate fasting or increases the postprandial glucose. Diabetes is characterized by high glucose due to a disturbance in insulin secretion, resistance to insulin, or both. Diabetes mellitus can occur when there is an inadequate disposition index or normal constant insulin secretion times insulin sensitivity; therefore, hyperglycemia cannot be prevented. The clinical presentation of these changes includes polydipsia, polyuria, polyphagia, and weight loss. Diabetes can be classified into type 1 and type 2. Type 1 diabetes mellitus (T1DM) is characterized by a severe lack of insulin because of decreased secretion of insulin. In contrast, type 2 diabetes mellitus is characterized by insulin resistance, which is usually caused by obesity, growth hormone, and cortisol. The most common type of diabetes in children is T1DM, in which there is a deficiency of insulin secretion from beta cells [1,2]. According to the Indonesian Pediatric Society data, there were 1,220 children with T1DM in Indonesia in 2018. However, many cases remain undiagnosed [3,4]. In 2017, 71% of children were only diagnosed with T1DM when they came to a healthcare facility with Diabetic Ketoacidosis [4].
T1DM is not curable, but can be managed properly to improve patients' quality of life (QoL). Treating a child with diabetes mellitus requires consideration of the child’s lifestyle, and an integrated approach involving the patient, family, doctor, and nutritionist. The patient should be followed frequently for assessment of growth and development, glycemic control, education, complications, problems affecting diabetes, and overall health. The goal of diabetes therapy, especially in T1DM, is to achieve a Hemoglobin A1c (HbA1c) and plasma glucose that are close to normal and to minimize the severe complication events (such as hypoglycemia and diabetic ketoacidosis). The International Society for Pediatric and Adolescent Diabetes recommends targeting an HbA1c of <7%, Meanwhile the American Diabetes Association treatment guidelines recommend a target HbA1c of <7.5% across all groups [5,6]. Treatment of diabetes mellitus includes insulin, blood glucose monitoring, medical nutrition therapy, and exercise. One of the dietary managements of diabetes mellitus is to count one's carbohydrate intake. Carbohydrates, an integral source of energy, are counted in order to adjust a patient’s insulin intake and ultimately to maintain the blood glucose level [1,2].
Carbohydrate counting is a meal-planning tool for patients with type 1 diabetes mellitus treated with a basal bolus insulin regimen. It is tailored based on awareness of carbohydratecontaining foods and their effect on blood glucose. The dose of insulin required is determined based on the total carbohydrates consumed with each meal, and the insulin-to-carbohydrate ratio (ICR). The current guidelines recommend algorithms for prandial insulin to be based on the amount of carbohydrate consumed during a meal.7)
The aim of this study is to measure the efficacy of HbA1c reduction with carbohydrate counting.
The systematic review and meta-analysis is conducted by adhering to the preferred reporting items for systematic review (PRISMA) [8]. This systematic review did not require patient consent or Research Ethics Committee approval.
Database searches were conducted according to the PRISMA guidelines. Eligible studies were selected from several databases such as MEDLINE using several search managers, such as PubMed, Scopus, ScienceDirect, and ProQuest. The search term used was formed using the PICO framework (P: patient or problems; I: intervention being considered; C: comparison intervention; O: outcome measurements). The focus of our search was studies implementing carbohydrate counting as an intervention in pediatric (<18 years old) type 1 diabetes mellitus patients (<18 years old). The PICO criteria can be found in Supplementary Material 1.
The available PICO criteria were then used for search queries and keywords, which can be seen in Supplementary Material 2. The exploded keywords were included, as were MESH terms for MEDLINE and modified truncation according to the different search platforms.
The literature search was followed by a screening process based on the predetermined inclusion and exclusion criteria. Original studies on carbohydrate counting in T1DM pediatric patients that were published within the last 10 years were included. This includes randomized or nonrandomized controlled trials, and cohort or cross-sectional studies. Studies were excluded if they were not published in English, had a nonretrievable full text, or were commentaries, reviews, or letters/correspondences.
A quality assessment of the included studies was undertaken based on the study designs. We used the Cochrane risk of bias (RoB) 2.0 for trial studies and the JBI Critical Appraisal Checklist for observational and cross-sectional studies [9]. In RoB 2.0, the quality of included studies was assessed according to the following 5 appraisal elements: randomization process, deviations from the intended interventions, missing outcome data, measurements of the outcome, and selection of the reported results. Studies were then scored as low risk, some concerns, and high risk. Meanwhile, in the JBI Critical Appraisal Checklist, the quality assessment items are population similarity, the validity of exposure, confounding factors, measurements of the outcome, follow-up, and statistical analysis [10]. The studies then are appraised as included, excluded, and requiring further information.
All reviewers independently screened all of the titles from the search and excluded studies that were irrelevant. Following this, all the reviewers independently screened the titles and abstracts using an eligibility checklist. Potentially eligible texts were retrieved for final selection. We included studies that met our inclusion criteria. Any discrepancies in the extracted data were discussed by all reviewers.
The following data were extracted: (1) general information (author, title, year of publication); (2) study characteristics (study design, number of samples); (3) intervention and setting (methods of carbohydrate counting, follow-up period, study location); (4) outcome data (baseline and follow-up measure). The following outcome data of the glycemic index control were collected: HbA1c changes, nutritional outcomes (low-density lipoprotein [LDL] and body mass index [BMI] changes), and QoL analysis.
To summarize the effect of carbohydrate counting on type 1 diabetes, we measured the outcome by HbA1c concentration, nutritional outcomes (changes in BMI and LDL), and QoL.
The software Revman 5.4.1 was used to analyze the data. The Generic Inverse Variance with Random Effects (DerSimonian-Laird) method was used for the analysis using the mean difference as the effect measure, with 95% confidence intervals (ICs). The analysis was then extrapolated into a forest plot.
The flowchart for the study selection can be seen in Fig. 1. The literature search yielded 1,693 articles on the initial hit. Four reviewers independently screened titles and abstracts. Of the 36 articles screened, 34 articles were found to be eligible. Of those, 25 studies were excluded because their outcomes and study designs were not suitable. After the screening process, we included 9 studies based on the eligibility criteria.
The included studies (Table 1), were comprised of 4 randomized controlled trials, one prospective controlled trial, one prepost trial, and 3 observational studies (prospective crosssectional and retrospective longitudinal study), with a total of 606 participants [11-19]. All of the studies were conducted at various locations within Europe and the United States. The follow-up or trial periods for each study are different, ranging from 3 months to 2 years. Most studies used basic carbohydrate counting, which involves basic calculations on the consumed carbohydrate daily. In contrast, one study used advanced carbohydrate counting, while another study used a carbohydrate counting application, which calculates the carbohydrate consumption automatically. Advanced carbohydrate counting not only adjusts the amount and time of carbohydrate consumption (like basic carbohydrate counting), but also regulates the number of grams of carbohydrates adjusted to the insulin dose based on the ICR. Out of 606 participants, there were 440 participants in the carbohydrate counting group and 166 in the control group. Three studies did not use a control group due to their observational method, while one study used its whole population on a pre-post trial. Therefore, the first period of the trial was used as the control outcome, while the second period of trial was used as the interventional outcome. Most studies classified the sample into 2 different groups (carbohydrate counting and control). However, Enander et al. [17] used 3 groups, with the interventional group divided into manual carbohydrate counting and calculated carbohydrate counting (using software or applications).
The RoB assessment was conducted with Cochrane RoB 2.0 for randomized studies and JBI Critical Appraisal Tool for nonrandomized studies [9,10]. The results can be seen in both Supplementary Materials 3 and 4.
With regard to the RoB for randomized studies, 4 of 5 studies that were assessed were considered to have low RoB. However, the study by Marigliano et al. [18] was assessed to have some concern on the overall risk assessment. In the study by Marigliano et al. [18], the randomization process was deemed unclear due to a lack of relevant information. Their methods only mentioned that there was a random assignment using 2-group randomized block design on both groups. Furthermore, the outcome measurement domain was deemed concerning due to the unblinded nature of the data analysis. In the study by Gökşen et al. [11], the outcome measurement and data selection domains were deemed concerning due to multiple eligible outcome measurement methods. However, all studies were deemed eligible to be included in this review.
The RoB assessment for the nonrandomized studies was conducted using the JBI Critical Appraisal Tool with an assessment of the research population, recruitment process, exposure measurement, outcome measurement, follow-up period, and appropriate statistical analysis. In the 4 studies assessed, all aspects were deemed to be clear. Therefore, the 4 studies were eligible for inclusion.
The data synthesis of the included studies is summarized in Table 2. The recorded outcomes include the HbA1c changes and nutritional outcomes (LDL and BMI). The effect was measured using the mean difference. The authors also recorded the QoL assessment, if available. Notable outcomes of each study were also recorded if the authors deemed it necessary to be included. All the studies comparing the carbohydrate counting group and a control group showed a greater reduction in the HbA1c in the carbohydrate counting group than in the control group. Most studies reported an increase in HbA1c at the end of the followup period in the control group. Enander et al. [17] also reported the difference between the manual and calculated carbohydrate counting methods; they found that manual carbohydrate counting led to less increase in the HbA1c than did calculated carbohydrate counting. (0.1±0.39 vs. 0.4±0.34). Meanwhile, the studies that did not have a control group reported a 0.5% to 1% reduction in the HbA1c at the end of the follow-up period.
The quantitative analysis of the glycemic index control included 6 studies, comparing the HbA1c changes between the carbohydrate counting group and its control counterpart [11-14,16,17]. The meta-analysis showed the cumulative effect on the mean difference of -0.55 (95% CI, -0.81 to -0.28; P-value <0.001). While all studies similarly found that the mean difference reduction favored the interventional group, the heterogeneity analysis showed an I2 value of 88%, which implies high heterogeneity between the studies (Fig. 2).
The nutritional outcomes extracted from the included studies were the LDL cholesterol changes and BMI changes in both groups. Only 5 studies reported the changes in LDL values [11,13,14,18,19], while only 4 studies reported BMI changes [11,16-18]. Gökşen et al. reported a lower increasing level of LDL concentration in the intervention group as compared to that in the control group (mean differences [MD], 15.26±5.81 vs. 18.22±6.17) after 2 years of follow-up. Bayram et al. reported a higher reduction of LDL in the carbohydrate counting group than in the control group (MD, -3.68±6.49 vs. -2.13±5.98) [11,14]. However, Dalsgaard et al. [13] reported the opposite result with a higher reduction of LDL in the control group than in the intervention group (MD, -1.39±5.31 vs. -7.20±5.29) [13]. The quantitative analysis of LDL changes reported no significant difference between the interventional and control groups with a cumulative mean difference of 0.53 (95% CI, -5.62 to -0.30), and high heterogeneity of 95% (Fig. 3). This outcome was most certainly caused by the different results in Dalsgaard et al.’s study [13].
The BMI change outcomes were quite similar across the studies that reported it, with a lower increase or decrease in BMI in the control group than in the intervention group. However, Gökşen et al. [11] reported a higher BMI increase in the control group in the first year of the study, while the opposite happened in the second year of observation.
Donzeau et al. [16] was the only group to assess QoL. Donzeau et al. [16] reported higher scores in the carbohydrate counting group than in the control group based on the DISABKIDS, KIDSCREEN, and DSQOL scoring. The scoring assesses the children's physical fitness, ability to study and make social connections, eagerness to play, and high aspiration toward the future. Carbohydrate counting is beneficial in the maintenance of QoL in diabetic children.
Our meta-analysis of the effect of carbohydrate counting on glycemic control in T1DM patients generally demonstrated a reduction in the HbA1c with carbohydrate counting. In the quantitative cumulative analysis, a mean difference of -0.55 (95% CI: -0.81 to -0.28, P<0.0001) reflected a significant reduction in the HbA1c level of T1DM patients who underwent carbohydrate counting. All of the included studies also reported similar results, with the exception of Enander et al. [17], who found insignificant differences despite the lower level of HbA1c found in the carbohydrate counting group.
Similar results have been reported by other studies with different populations of diabetes mellitus patients. For instance, Schmidt et al. [20] conducted a systematic review of adult patients with T1DM, and found a 0 to 13 mmol/mol (1.2%) reduction in the HbA1c and significant decrease in hypoglycemia events in the carbohydrate counting group compared to those in the control group. Meanwhile, Fu et al. [21] conducted a meta-analysis on the use of advanced carbohydrate counting on diabetes mellitus patients, with an HbA1c reduction of -0.35% compared to that of any other dietary method or education used.
However, contrary results were also reported by several authors, such as Kalergis et al. [22], who reported nonsignificant changes in glycemic control and QoL with the use of carbohydrate counting. Meanwhile, Gilbertson et al. [23] reported similar results on the glycemic control aspect; however, the study found a significant decrease in hypoglycemia incidents in the intervention group compared to that in the control group. The contrary results might be explained by the inaccuracy of carbohydrate counting among patients, as reported by Meade et al. [24], who found an accuracy rate of 59% on the carbohydrate counting method used by patients. Therefore, the effect might not be optimal to improve glycemic control in these DM patients.
In the meta-analysis, the selected studies demonstrated different results when looking at the outcome of changes in LDL and BMI. The Gökşen and Bayram study, which assessed LDL output, showed a greater LDL reduction in the intervention group (or carbohydrate counting group) than in the control group. This finding is in contrast to the study by Dalsgaard et al., who found a greater LDL reduction in the control group than in the intervention group [11,13,14]. The 3 inclusion studies in the meta-analysis had a cumulative mean difference of 0.53 (95% CI, -5.62 to -0.30), which was not significant between the intervention and control groups. Kostopoulou et al. [19] found that there was no significant change in the lipid profile of LDL, high-density lipoprotein, triglycerides, and cholesterol after using the carbohydrate counting intervention. The LDL levels before and after the intervention were 84 mg/dL and 87 mg/dL, respectively (P=0.937). In contrast, Abaci et al. [25], reported changes in LDL after one year of carbohydrate counting with a significant decrease from 92.75 mg/dl to 71.7 mg/dL (P=0.036).
As with changes in LDL, changes in BMI in selected studies did not show a significant outcome. In Abaci et al. [25] did not find a significant change in BMI with the intervention changes (P=0.066); the BMI changes with standard deviation values before and after the intervention were 1.05 and 1.16, respectively. In the study by Trento et al. [26], the effect of carbohydrate counting on changes in BMI was also not significant. The baseline BMI in the intervention group was 24.4±2.6 and after the intervention for 30 months, 23.4±5.3.
Only Donzeau et al. [16] assessed QoL in T1DM patient who underwent carbohydrate counting. Their study showed that the carbohydrate counting group had higher scores (than did the control group) based on the QoL scoring with DISABKIDS, KIDSCREEN, and DSQOL. These scorings assess the children's physical fitness, ability to study, ability to make social connections, eagerness to play, and high aspiration toward the future. This study concludes that carbohydrate counting is beneficial to maintain the QoL of diabetic children. Tascini et al. [7] also evaluated QoL using the Diabetes Quality of Life for Youth scale. This group also reported that carbohydrate counting has a positive effect on improving QoL. A study from Robart et al. [27] also shows a better QoL for children with T1DM using carbohydrate counting compared to those who do not do carbohydrate counting.
This systematic review and meta-analysis have several strengths. To our knowledge, this study is the first to review the implementation of carbohydrate counting in glycemic outcome, nutritional outcome, and QoL improvement in the pediatric population. The focus on the pediatric population might show the impact of the dietary method on children despite the difficulties in managing a proper menu. Our inclusion criteria ensured that only studies with suitable study designs were eligible. We accepted 5 randomized studies and 4 nonrandomized studies in a variety of study locations. In addition, we assessed 9 studies independently with the results of 9 studies having low RoB.
There were some methodological issues with regard to the sample size included in this review. Only 606 samples were included in the 9 studies included. This sample size limits our findings in terms of accuracy. Another limitation is the nonsignificant results in nutritional outcomes, particularly due to the heterogenous nature of the cumulative data due to different conclusions in each study. The heterogenous data are also not fully explained, except for differences in the study population. Therefore, proper conclusions might be limited based on these data. Despite these limitations, this review provides useful information for future interventions for T1DM in pediatric population.
In conclusion, this comprehensive meta-analysis demonstrates that carbohydrate counting produced a significant reduction in the HbA1c levels of T1DM patients, but not a significant result in nutritional outcomes (LDL and BMI) and QoL. Therefore, we recommend the implementation of carbohydrate counting for reducing HbA1c levels in T1DM patients. Larger clinical trials are warranted to validate our findings in this meta-analysis.
Supplementary material
Supplementary Materials can be found via https://doi.org/10.6065/apem.2244242.121.
Supplementary Material 1.
PICO criteria used in the systematic review
Supplementary Material 2.
Search queries used on each search manager for the literature search.
Supplementary Material 3.
Cochrane risk of bias 2 risk of bias assessment results on five randomized controlled trials studies. There’s a total of five domains assessed in the assessment, including: the randomization process (D1), deviations from intended interventions (D2), missing outcome data (D3), measurements of the outcome (D4), and selection of reported results (D5). HbA1c, hemoglobin A1c.
Supplementary Material 4.
JBI critical appraisal tool on four non-randomized studies (cross-sectional or cohort).
Notes
ACKNOWLEDGMENTS
The authors would like to acknowledge all of the authors from the included studies who have pursued research in this field.
Fig. 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart on the literature search and screening process.
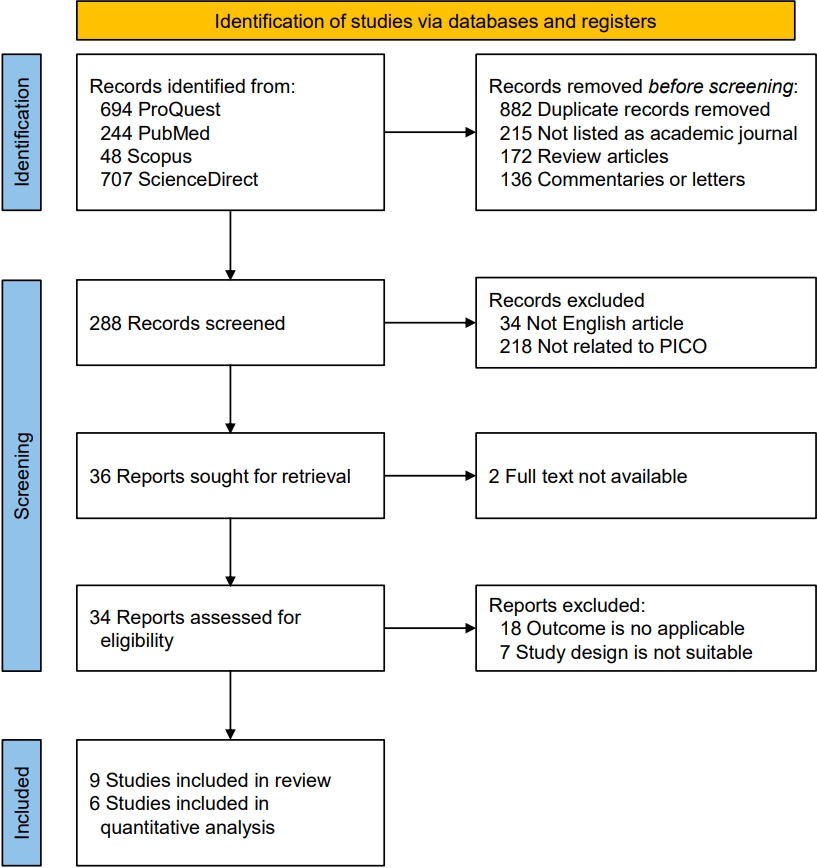
Fig. 2.
Forest plot of hemoglobin A1c changes. SE, standard error; CI, confidence interval; df, degrees of freedom.
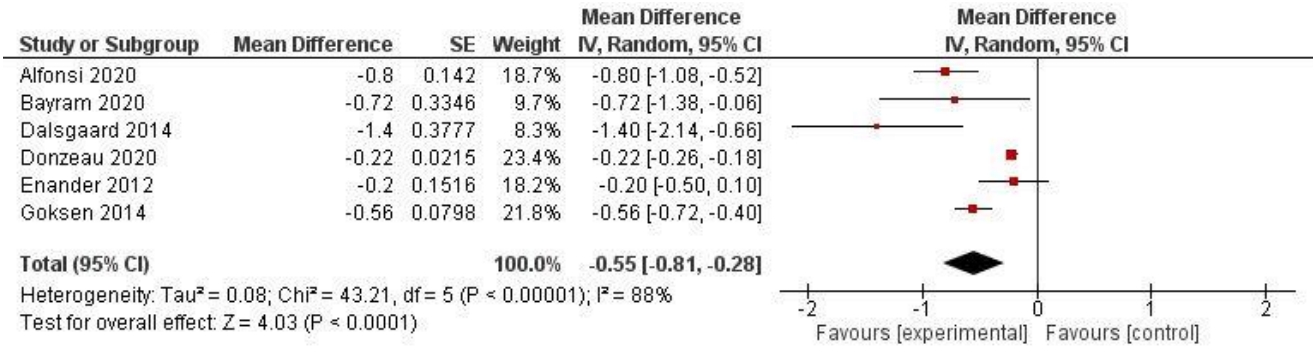
Fig. 3.
Forest plot of low-density lipoprotein changes. SE, standard error; CI, confidence interval; df, degrees of freedom.
Table 1.
Characteristics of the included studies
| Study | Study population | Study design | Follow-up (trial) period | Study location; settings | N samples included (CC group; control group) | Carbohydrate counting method |
|---|---|---|---|---|---|---|
| Gökşen et al. [11] (2014) | T1DM children & adolescents aged 7–18 years old | Randomized controlled trial (RCT) | 2 Years | Turkey; Hospital | 84 (52; 32) | Basic carbohydrate counting |
| Bayram et al. [14] (2020) | Children and adolescents with TD1M aged 2 to 18 years old; receiving intensive insulin therapy were trained and followed for 6 months | Prospective cross-sectional | 6 Months | N/A; Hospital | 53 (27; 26) | Basic carbohydrate counting |
| Kostopoulou et al. [19] (2019) | TD1M patients aged 2 to 23 years old | Pre-post trial | 4 Months | Greece; Hospital | 35 (CC group only) | Basic carbohydrate counting |
| Fortins et al. [15] (2019) | 7–16 years old; diagnosis of T1DM 1 year prior; absence of autoimmune disease, genetic syndromes, sickle cell anemia, and renal failure; nonuse of steroid and insulin pump | Analytical cross-sectional | N/A (cross-sectional) | Rio de Janeiro, Brazil; Hospital | 120 (CC group only) | Basic carbohydrate counting |
| Donzeau et al. [16] (2020) | <18 Years old; diagnosis of T1DM at least 1 year previously; treatment with insulin pump at least 6 months; HbA1c <9%; not in remission phase | RCT | 12 Months | French; Pediatric centers | 87 (40; 47) | Advanced carbohydrate counting |
| Enander et al. [17] (2012) | <18 Years old; had been treated with CSII >6 months; not in remission phase | RCT | 12 Months | Lidkoping, Sweden; Pediatric centers | 40(Manual CC: 12; Calculated CC: 14; | Basic carbohydrate counting |
| Alfonsi et al. [12] (2020) | T1DM patients aged 8–18 years old | Pilot RCT | 3 Months | Canada; Hospital | 44 (22; 22) | Carbohydrate counting application (iSpy) |
| Dalsgaard et al. [13] (2014) | T1DM children and adolescents | Retrospective longitudinal study | 1 Year | Rio de Janeiro, Brazil; Hospital | 93 | Basic carbohydrate counting |
| Marigliano et al. [18] (2013) | T1DM aged 7–14 years old | Prospective clinical trial | 18 Months | Verona, Italy; Hospital | 50 (25; 25) | Basic carbohydrate counting |
Table 2.
The outcomes of the included studies
| Study | N Included Samples (CC; control) |
HbA1c changes (MD;SD) |
Nutritional outcomes |
QoL outcomes |
Miscellaneous | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC group | Control group | LDL changes on CC group | LDL changes on Control group | BMI changes on CC group | BMI changes on Control group | QoL improvement in CC group | QoL improvement in control group | |||
| Gökşen et al. [11] (2014) | 84 (52;32) | 1 Year: -0.52;0.19 | 1 Year: -0.42;0.34 | 1 Year: +0.68;4.92 | 1 Year: +10.45;6.09 | 1 Year: +0.65 | 1 Year: +0.74;0.87 | N/A | N/A | |
| 2 Year: -0.23;0.24 | 2 Year: +0.33;0.41 | 2 Year: +15.26;5.81 | 2 Year: +18.22;6.17 | 2 Year: +1.20 | 2 Year: +0.91;0.88 | |||||
| Bayram et al. [14] (2020) | 53 (27;26) | -0.22;1.05 | +0.50;1.36 | -3.68;6.49 | -2.13;5.98 | N/A | N/A | N/A | N/A | Significant decrease of BMI-SDS |
| Kostopoulou et al. [19] (2019) | 35 | -0.50;0.19 | N/A | 3.0;4.55 | N/A | N/A | N/A | N/A | N/A | |
| Fortins et al. [15] (2019) | 120 (CC group only) | -1.0;0.14 | N/A | N/A | - | - | - | - | - | |
| Donzeau et al. [16] (2020) | 87 (40;47) | Overall: -0.17;0.10 | Overall: +0.05;0.1 | N/A | N/A | BMI z-score was 0.8 SD 0.8 at study entry and 0.7 SD 1.0 at 12 months, there are no significant differences | Significantly higher scores on CC group (DISABKIDS, KIDSCREEN, DSQOL scores) as compared to control group | Hypoglycemia incident: 4.0;3.6 vs. 5.1;4.6 for CC group. | ||
| Severe hypoglycemia is considered similar in both groups. | ||||||||||
| DKA: 3% vs. 2% for CC group | ||||||||||
| Enander et al. [17] (2012) | 40 (Manual CC: 12; Calculated CC: 14; 14) | Manual CC group: 3 mo: -0.30;0.39, 12 mo: +0.1;0.39 Calculated CC group: 3 mo: +0.1;0.29, 12 mo: +0.4;0.34 | 3 mo: +0.1;0.36 | N/A | N/A | No changes after 12 months | 12 mo: -0.2;0.39 (BMI-SDS value) | N/A | N/A | |
| 12 mo: +0.3;0.38 | ||||||||||
| Alfonsi et al. [12] (2020) | 44 (22;22) | -0,35;0.50 | +0,45;0.44 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Dalsgaard et al. [13] (2014) | 93 | Y1: -0.80;1.32 | Y1: +0.1;1.33 | Y2: -1.39;5.31 | Y2: -7.20;5.29 | N/A | N/A | N/A | N/A | |
| Y2: -1.30;1.31 | Y2: +0.1;1.36 | |||||||||
| Marigliano et al. [18] (2013) | 25 | -0.58;0.21 | N/A | -0.70;5.83 | N/A | 1.40;0.72 | N/A | N/A | N/A | |
References
1. Marcdante KJ, Kliegman RM. Diabetes mellitus. In: Nelson textbook of pediatrics. 21st ed. Singapore: Elsevier Inc., 2021:572-9.
2. Sperling M, Tamborlane M, Majzoub JA, Menon RK, Stratakis CA. Diabetes mellitus. In: Sperling M, Majzoub JA, Menon RK, Stratakis CA, editors. Sperling pediatric endocrinology. 5th ed. Philadelphia (PA): Elsevier-Saunders, 2020:846-9.
3. Lupsa B, Inzucchi S. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome. In: Loriaux L, editor. Endocrine emergencies: recognition and treatment [Internet]. Totowa: Humana Press; 2014 [cited 2022 Oct 19]. p. 15–30. Available from: https://books.google.com/books/about/Endocrine_Emergencies.html?id=A4a8BAAAQBAJ.
4. Pulungan AB, Annisa D, Imada S. Type-1 diabetes mellitus in children: Indonesian situation and management. Sari Pediatri 2019;20:392–400.
5. Besser REJ, Bell KJ, Couper JJ, Ziegler AG, Wherrett DK, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 2022;23:1175–87.

6. American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes 2022;40:10–38.




7. Tascini G, Berioli MG, Cerquiglini L, Santi E, Mancini G, Rogari F, et al. Carbohydrate counting in children and adolescents with type 1 diabetes. Nutrients 2018;10:109.



8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.



9. Higgins J, Thomas J, Chandler J, Cumpston M. Cochrane handbook for systematic reviews of interventions. 2nd ed. Hoboken: The Cochrane Collaboration; 2019:1-539.
10. Critical Appraisal Tools | JBI [Internet]. JBI. 2022 [cited 2022 Oct 19]. Available from: https://jbi.global/critical-appraisaltools.
11. Gökşen D, Atik Altınok Y, Ozen S, Demir G, Darcan S. Effects of carbohydrate counting method on metabolic control in children with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol 2014;6:74–8.


12. Alfonsi JE, Choi EEY, Arshad T, Sammott SS, Pais V, Nguyen C, et al. Carbohydrate counting app using image recognition for youth with type 1 diabetes: pilot randomized control trial. JMIR Mhealth Uhealth 2020;8:e22074.



13. Dalsgaard H, Saunders C, Padilha Pde C, Luescher JL, Szundy Berardo R, Accioly E. Glycemic control and lipid profile of children and adolescents undergoing two different dietetic treatments for type 1 diabetes mellitus. Nutr Hosp 2014;29:547–52.

14. Bayram S, Kızıltan G, Akın O. Effect of adherence to carbohydrate counting on metabolic control in children and adolescents with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab 2020;25:156–62.




15. Fortins RF, Lacerda EMA, Silverio RNC, do Carmo CN, Ferreira AA, Felizardo C, et al. Predictor factors of glycemic control in children and adolescents with type 1 diabetes mellitus treated at a referral service in Rio de Janeiro, Brazil. Diabetes Res Clin Pract 2019;154:138–45.


16. Donzeau A, Bonnemaison E, Vautier V, Menut V, Houdon L, Bendelac N, et al. Effects of advanced carbohydrate counting on glucose control and quality of life in children with type 1 diabetes. Pediatr Diabetes 2020;21:1240–8.



17. Enander R, Gundevall C, Strömgren A, Chaplin J, Hanas R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatr Diabetes 2012;13:545–51.


18. Marigliano M, Morandi A, Maschio M, Sabbion A, Contreas G, Tomasselli F, et al. Nutritional education and carbohydrate counting in children with type 1 diabetes treated with continuous subcutaneous insulin infusion: the effects on dietary habits, body composition and glycometabolic control. Acta Diabetol 2013;50:959–64.



19. Kostopoulou E, Livada I, Partsalaki I, Lamari F, Skiadopoulos S, Rojas Gil AP, et al. The role of carbohydrate counting in glycemic control and oxidative stress in patients with type 1 diabetes mellitus (T1DM). Hormones (Athens) 2020;19:433–8.



20. Schmidt S, Schelde B, Nørgaard K. Effects of advanced carbohydrate counting in patients with type 1 diabetes: a systematic review. Diabet Med 2014;31:886–96.


21. Fu S, Li L, Deng S, Zan L, Liu Z. Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: a systematic review and meta-analysis. Sci Rep 2016;6:37067.




22. Kalergis M, Pacaud D, Strychar I, Meltzer S, Jones PJ, Yale JF. Optimizing insulin delivery: assessment of three strategies in intensive diabetes management. Diabetes Obes Metab 2000;2:299–305.


23. Gilbertson HR, Brand-Miller JC, Thorburn AW, Evans S, Chondros P, Werther GA. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes Care 2001;24:1137–43.



25. Abacı A, Ataş A, Ünüvar T, Böber E, Büyükgebiz A. The effect of carbohydrate counting on metabolic control in patients with type 1 diabetes mellitus. Gülhane Tıp Dergisi 2009;51:1–5.
- TOOLS
- Related articles in APEM







