The utilization of basal luteinizing hormone in combination with the basal luteinizing hormone and follicle-stimulating hormone ratio as a diagnostic tool for central precocious puberty in girls
Article information
Abstract
Purpose
Intravenous gonadotropin-releasing hormone (IV GnRH) testing is the gold standard for confirming a central precocious puberty (CPP) diagnosis. However, this test is not widely available commercially. Therefore, our study aim was to establish cutoff values for basal gonadotropin level and gonadotrophin responses to a 100-μg subcutaneous IV GnRH test that can distinguish between CPP and premature thelarche (PT) to discover a simple method to detect CPP.
Methods
Girls between the ages of 6 and 8 years who attended the pediatric endocrinology outpatient clinic at our tertiary hospital between 2019 and 2022 were included in this study. They were evaluated for breast development, and a subcutaneous 100-μg GnRH test was administered by measuring the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels in blood samples at baseline and then 30, 60, 90, and 120 minutes after injection. CPP is characterized by increased height velocity, advanced bone age, and progression of breast development. The cutoff value for diagnosis of CPP was determined using a receiver operating characteristic (ROC) analysis.
Results
In 86 Thai girls (56 with CPP and 30 with PT), the ROC analysis showed 71.4% and 100% sensitivity and specificity, respectively, for basal LH (cutoff ≥ 0.2 IU/L) plus the basal LH/FSH ratio (cutoff ≥ 0.1). The optimal cutoff values for peak LH (cutoff ≥ 7 IU/L) demonstrated a sensitivity of 94.6% and a specificity of 100%, whereas the LH value at 30 and 60 minutes after injection (cutoff ≥ 6 IU/L) demonstrated sensitivities of 92.9% and 94.6% and a specificity of 100%, respectively
Conclusions
Combining the basal LH (cutoff: 0.2 IU/L) and the basal LH/FSH ratio (cutoff: 0.1) can easily and cost-effectively diagnose CPP in a girl in breast Tanner stage II.
Highlights
· The integration of basal hormone (LH) and basal LH/follicle-stimulating hormone ratio presents a cost-effective approach for diagnosing central precocious puberty in a girl with breast Tanner stage II.
· Gonadotropin-releasing hormone stimulation test optimal cutoff values for peak LH (cutoff ≥7 IU/L) demonstrated a sensitivity of 94.6% and a specificity of 100%.
Introduction
Puberty is the transition from sexual immaturity to sexual maturity or the development of secondary sexual characteristics. In girls, the first sign of puberty onset is breast development, followed by a growth spurt and menarche [1,2]. Precocious puberty is usually identified when breast development occurs in girls before the age of 8. Central precocious puberty (CPP) is caused by early activation of the hypothalamic-pituitary-gonadal (HPG) axis [3,4]. CPP has numerous negative consequences, including early menarche, a shorter final height due to the influence of estradiol on longitudinal bone growth, and stress-related psychosocial issues [5,6]. Early diagnosis and therapy can help to reduce these problems [7,8].
Premature thelarche (PT) is defined as isolated breast development in the absence of other clinical signs of puberty [9]. PT is regarded as a normal variant of development and is not considered pathological [10]. In contrast to CPP patients, the growth rate in individuals with PT is normal, their bone age (BA) is not advanced, and their gonadotropin and estradiol levels remain at prepubertal levels [11]. The basal and stimulated luteinizing hormone (LH) values are diagnostic tests that demonstrate the activation of the HPG axis and enable differentiation between CPP and PT [12,13]. The gonadotropin-releasing hormone (GnRH) stimulation test is the gold standard for differentiating CPP from PT [14]. As a result, many studies have attempted to determine the cutoff values that provide the best sensitivity and specificity in distinguishing CPP cases from PT cases by evaluating basal and stimulated LH levels using various measurement methods [15]. In Thailand, GnRH stimulation tests use 100 μg of triptorelin subcutaneously, which is a different protocol from that used in other countries [13].
This study aimed to determine the laboratory test with the highest diagnostic accuracy for CPP and to compare the clinical, anthropometric, and laboratory findings of patients who presented to an outpatient pediatric endocrinology clinic with premature breast development and underwent subcutaneous triptorelin testing.
Materials and methods
1. Participants
This was a prospective observational study that included a sample of 86 Thai girls aged 6–8 years who were evaluated for breast Tanner stage II before the age of 8 and underwent the subcutaneous triptorelin test in the pediatric endocrinology outpatient clinic at King Chulalongkorn Memorial Hospital between October 2019 and June 2022. Exclusion criteria were girls with peripheral precocious puberty due to external causes, such as an adrenal tumor, an ovarian cyst, an ovarian tumor, or exogenous hormonal substances.
The demographic data collected included chronological age (CA; years); age at breast development onset (years); BA (years); breast Tanner stage; pubic hair Tanner stage; height (standard deviation score [SDS]); weight (SDS); body mass index (BMI, SDS); and serum LH, FSH, and estradiol levels at the time of the subcutaneous triptorelin test. The development of breasts and the appearance of pubic hair were rated according to the Tanner stage based on inspection and palpation. The National Standard Growth Curve of Thailand's Ministry of Public Health was used to determine the SDS for height, weight, and BMI (Department of Health, Ministry of Public Health, Thailand; reference for weight and height in Thai youth from the ages of 1 day to 19 years; Bangkok, Thailand 2020). The Greulich and Pyle technique was utilized by a pediatric endocrinologist to determine patient BA [16]. All participants were followed until the age of 8.5–9.5 years.
2. GnRH stimulation test
The physician injected 100 μg of triptorelin acetate (IPSEN Pharma Biotech, Paris, France) subcutaneously into the right lateral upper arm between 8:00 AM and 10:00 AM Blood samples were collected for LH, FSH, and basal estradiol measurements at 0, 30, 60, 90, and 120 minutes. The test was carried out by measuring the serum LH, FSH, and estradiol levels at baseline (between 8:00 AM and 9:00 AM). LH and FSH were obtained using Elecsys Assay commercial kits (Roche Diagnostics GmbH, Mannheim, Germany) and analyzed with a Cobas 6000 and 601 automated system using the electrochemiluminescence method (ECLIA) (Roche Diagnostics GmbH). The method's minimum detection value is 0.1 IU/L for both hormones. LH has an approximately 2% intra- and interassay variability, while FSH has 2.8% intraassay variability and 4.5% interassay variability. Estradiol was measured using the Elecsys Estradiol III Assay (Roche Diagnostics GmbH) through ECLIA. The method's lower limit value is 5 pg/mL, and its intra- and interassay variability values are up to 6.7% and 10.6%, respectively. The LH/FSH ratio at baseline as well as that after triptorelin administration were both calculated.
Based on the results of the subcutaneous triptorelin test, the sample of girls was divided into 2 groups. Girls with peak LH level > 5 IU/L were categorized into the CPP group, while those with lower values were classified into the PT group [13]. All participants in the PT group were observed until they reached 8.5 years of age.
3. Statistical analysis
All analyses were performed using Stata 15.1 (Stata Corp., College Station, TX). To compare the demographic data and laboratory results for CPP and PT patients, the results were presented as the median with the interquartile range, and the Wilcoxon rank-sum test was used to analyze differences in continuous variables. A P-value of <0.05 was considered statistically significant. In cases of CPP, a receiver operating characteristic (ROC) curve analysis was used to determine the laboratory test cutoff value with the highest diagnostic accuracy. The empirical estimation of a cutoff point for a diagnostic test was made using the Liu method. All reported P-values were 2-sided. A Spearman correlation analysis was carried out to determine the correlations between the basal and peak LH, the basal and peak FSH, the basal and peak LH/FSH ratio, and the basal estradiol and basal LH. In addition, a logistic regression model was applied to identify the risk factors for CPP. A multivariate analysis was developed using a covariate with P<0.1 from the univariate and stepwise backward logistic regressions to select the final model.
4. Ethical statement
Informed consent was obtained from all participants and their parents, and they were provided with the study purpose and processes involved. The study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine at Chulalongkorn University (IRB No.1022/64) under the international guidelines for human research protection of the Declaration of Helsinki, the Belmont Report, the CIOMS Guidelines, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Results
Eighty-six girls participated in the study. During the follow-up period, patients with premature breast development who also demonstrated increased height velocity, progression of breast development, and advanced BA were diagnosed with CPP. The remaining patients were diagnosed with PT. The CPP group included 56 girls, while the PT group included the remaining 30 patients. The clinical characteristics and hormonal profiles of both groups are shown in Table 1. According to the demographic data, both age at the first visit and age of breast development were older in the CPP group than in the PT group. Although there was a significant age difference at the initial visit, there was no difference in age of breast development.
A comparison of anthropometric data revealed no statistically significant differences in weight SDS, height SDS, and BMI SDS between the 2 groups. In addition, our findings indicated that the BA at the first visit was 8.8 years (range, 8–11 years) in CPP patients and 7.8 years (range, 6.8–8.3 years) in PT patients, and that the BA/CA ratio and BA advancement (BA-CA) in the CPP group were significantly different from the PT group (Table 1). The CPP group demonstrated significantly increased levels of basal LH, LH at every time point, peak LH, basal FSH, peak FSH, basal estradiol, and basal and peak LH/FSH ratios (P<0.05). The peak LH in CPP rapidly rises between 30 and 60 minutes after initiation of the test (Fig. 1).
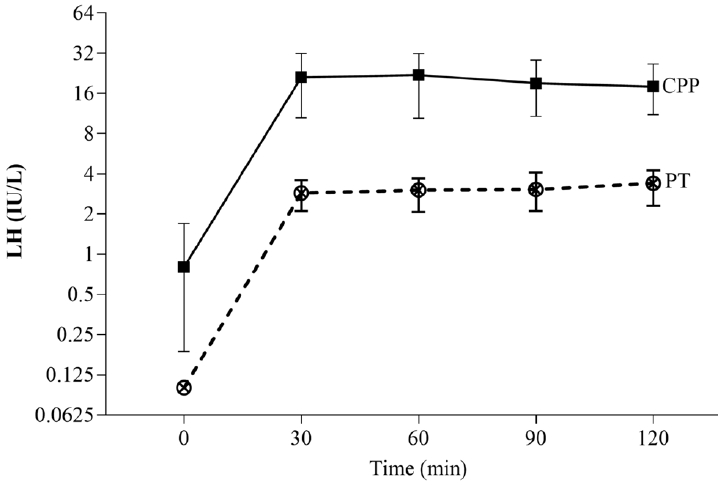
A comparison of the serum luteinizing hormone (LH) concentrations with the elapsed time after 100 mcg subcutaneous triptorelin injection between the central precocious puberty (CPP) and the premature thelarche (PT) patients. Data are presented as the median and 95% confidence interval.
The ROC analysis revealed that peak LH (area under the curve [AUC]=0.973), LH at 30 minutes (AUC=0.964), and LH at 60 minutes (AUC=0.973) had the highest diagnostic accuracy for CPP (Fig. 2). The ideal cutoff value for peak LH (cutoff ≥7 IU/L) had a sensitivity of 94.6% and a specificity of 100%; LH at 30 minutes (cutoff ≥6 IU/L) had a sensitivity of 92.9% and a specificity of 100%, while LH at 60 min (cutoff ≥6 IU/L) had a sensitivity of 94.6% and a specificity of 100% (Table 2). The basal LH (cutoff >0.1 IU/L) had an AUC of 0.834, a sensitivity of 76.8%, and a specificity of 90%. The basal LH (cutoff ≥0.3 IU/L) had an AUC of 0.866, a sensitivity of 73.2%, and a specificity of 100%. The sensitivity and specificity of the combination of basal LH (cutoff ≥0.2 IU/L) and basal LH/FSH ratio (cutoff ≥0.1) were 71.4% and 100%, respectively (Fig. 3). A Spearman rank correlation revealed a significant positive correlation (P<0.01) between the basal and peak LH, the basal and peak FSH, the basal and peak LH/FSH ratio, and the basal estradiol and basal LH values.
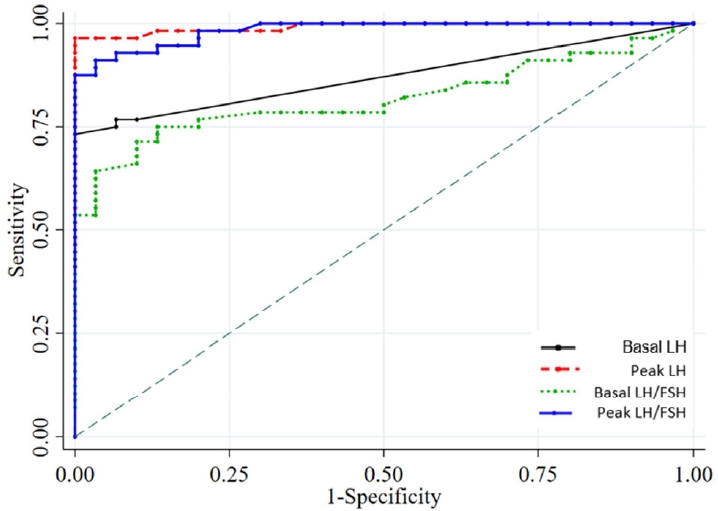
The area under the receiver operating characteristic (ROC) curve and the optimal cutoff point for the basal LH, peak LH, basal LH/FSH ratio, and peak LH/ FSH ratio to predict central precocious puberty. LH, luteinizing hormone; FSH, follicle-stimulating hormone.
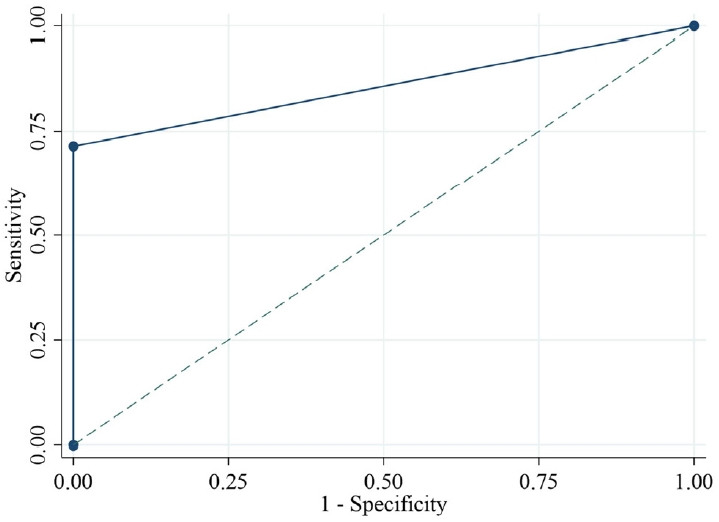
The area under the receiver operating characteristic (ROC) curve and the optimal cutoff point for a basal LH value >0.2 IU/L and a basal LH/FSH ratio ≥ 0.1 to predict central precocious puberty. LH, luteinizing hormone; FSH, folliclestimulating hormone.
To determine the risk factors associated with CPP, the findings indicated that CPP and PT differed statistically in certain characteristics. Indicators such as peak LH > 5 (adjusted odds ratio [aOR], 87.95; P=0.001), basal LH/FSH > 0.1 (aOR, 11.01; P=0.046), and peak LH/FSH ratio > 0.35 (aOR, 7.88; P=0.044) were evaluated using multivariate logistic regression.
Discussion
In the early stage of puberty, when a breast bud is the only manifestation, the progression of puberty in girls is not always continuous. It is often unclear whether the child has CPP or PT at this time [17]. Girls with CPP require prompt treatment to maintain normal height gains and menstrual timing [7]. This study demonstrated that auxological characteristics are poor predictors of CPP in girls before 8 years of age and should not be used to distinguish between PT and CPP. In addition, no single diagnostic laboratory method that could conclusively differentiate these 2 conditions from one another has been established. The gold standard for confirming CPP is the intravenous GnRH test. When administration of 100 μg of intravenous synthetic GnRH results in an increase in LH concentration from baseline to >5 IU/L, this result indicates activation of the HPG axis [18]. Nonetheless, this synthetic substance is unavailable in many countries, including Thailand. Therefore, basal gonadotrophin may be the initial test of choice for CPP, and 100 μg of a subcutaneous GnRH agonist (triptorelin) may serve as a suitable alternative for GnRH testing.
Our study also demonstrated that both basal LH and basal LH/FSH had a moderate degree of accuracy. The combination of basal LH and the basal LH/FSH ratio had a high level of specificity and was adequate for diagnosing CPP. In addition, our study demonstrated that the peak LH level and the peak LH/FSH ratio were adequate indicators of HPG axis activation following a subcutaneous triptorelin test. In girls with CPP, subcutaneous triptorelin significantly increased the LH level from 0.8 to 23.4 IU/L. The LH level in girls with PT increased from 0.1 to 3.5 IU/L. Consequently, the peak LH level after a subcutaneous triptorelin test can distinguish between girls with CPP and those with PT. The peak concentration of LH for patients with CPP occurred between 30 and 60 minutes after a subcutaneous injection of triptorelin, whereas previous studies have reported a peak LH level between 20 and 40 minutes after an intravenous injection of GnRH [19,20]. Therefore, subcutaneous triptorelin results in a delayed peak LH response compared to intravenous synthetic GnRH.
In terms of gonadotropin concentration, a statistically significant difference was discovered between the baseline values. The analysis of the ROC curve revealed levels ≥0.2 IU/L for basal LH and ≥0.1 for the basal LH/FSH ratio; their sensitivity and specificity profiles are shown in Table 2. Some authors have reported similar values and have suggested that baseline LH level is adequate to demonstrate activation of the HPG axis [12,21-23]. Our study indicated that the combination of a basal LH ≥0.2 IU/L and a basal LH/FSH ratio ≥0.1 had a high level of specificity and was sufficient for diagnosing CPP. Comparing the stimulated LH levels and peak LH/FSH ratio concentrations of CPP and PT revealed a statistically significant difference. In clinical practice, the LH peak has traditionally been used. A basal LH cutoff level ≥0.2 IU/L may also be utilized in the diagnosis of CPP. Nonetheless, the specificity of this single test alone was inferior to the combination of a basal LH ≥0.2 IU/L and a basal LH/FSH ratio ≥0.1. To determine the optimal stimulated values to use in clinical practice to maintain a high accuracy rate, the ROC curve established an LH cutoff value ≥6 IU/L at 30, 60, 90, and 120 min and an LH peak cutoff value ≥7 IU/L.
When beginning to investigate a diagnosis of CPP in patients based on clinical data and baseline LH level, we recommend that the baseline LH and basal LH/FSH ratio be determined. If the basal LH is ≥0.2 IU/L and the basal LH/FSH ratio is ≥0.1, this confirms the diagnosis with high specificity; if only the basal LH value is between 0.1 and 0.2 IU/L, a subcutaneous triptorelin test may be required. In a prior analysis of the LH response to a subcutaneous triptorelin test in patients with PT, most measurements were <4.0 IU/L [24]. These values were remarkably similar to those that have been widely reported in the published research as cutoff values for intravenous GnRH [17].
The present study has some limitations, including lack of an intravenous GnRH group due to the unavailability of this compound in Thailand. We concluded that basal LH ≥0.2 IU/L combination with a basal LH/FSH ratio ≥0.1 is effective for distinguishing girls with demonstrated activation of the HPG axis, allowing differentiation between CPP, which requires treatment, and PT, which only requires periodic clinical observation. Patients with CPP can also be diagnosed using a basal LH cutoff level ≥0.3 IU/L; an LH cutoff value ≥6 IU/L at 30, 60, 90, and 120 minutes; and an LH peak cutoff ≥7 IU/L after a subcutaneous triptorelin test, which is highly specific for distinguishing between CPP and PT.
In conclusion, a basal serum LH cutoff ≥0.2 IU/L in combination with a basal LH/FSH ratio ≥ 0.1 could be a simple and cost-effective approach for diagnosing CPP in girls with Tanner breast stage 2 prior to GnRH testing.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was funded by the Ratchadapiseksomphot Endowment Fund of Chulalongkorn University in Bangkok, Thailand (Grant No. 65/55).
Author contribution
Data curation: NC, SA; Formal analysis: WO, NN, VS; Funding acquisition: KS; Methodology: KS; Project administration: VS; Writing - original draft: NC; Writing - review & editing: KS
Acknowledgements
We would like to thank the patients, their families, pediatric residents, and pediatric endocrinology fellows for participating in the study.


