Effect of gonadotropin-releasing hormone agonist treatment on near final height in girls with central precocious puberty and early puberty
Article information
Abstract
Purpose
The aim of this study was to examine whether gonadotropin-releasing hormone (GnRH) agonist treatment is effective in preserving final height in patients with central precocious puberty (CPP) or early puberty (EP).
Methods
The medical records of 40 patients with CPP and 206 patients with EP who completed GnRH agonist treatment following diagnosis were analyzed retrospectively. Height and height standard deviation (height SDS) scores based on bone age (BA) were measured and calculated at baseline, after treatment completion, and at final follow-up to compare changes within and between groups. Predicted adult height (PAH) was estimated by the height corresponding to height SDS for BA in girls at 18 years 11 months of age based on the growth chart.
Results
PAH at baseline did not differ significantly between the CPP group (153.67±4.95) and the EP group (154.77±3.72). In the CPP group, PAH significantly increased at treatment completion (156.01±4.61) and at final follow-up (158.52±6.04) compared to baseline. In the EP group, PAH significantly increased at treatment completion (157.7±3.60) and at final follow-up (159.31±4.26) compared to baseline. The increase in PAH at all timepoints compared to baseline did not significantly differ between the CPP and EP groups.
Conclusions
Both CPP and EP groups had significantly greater PAH after treatment, with no difference in the amount of increase between groups. These results show that GnRH agonist treatment can help increase final height even in patients diagnosed with EP after the age of 8 years.
Highlights
· Gonadotropin-releasing hormone agonist treatment can help increase final height in both central precocious puberty and early puberty diagnosed after the age of 8 years.
Introduction
Central precocious puberty (CPP) is the onset of secondary sex characteristics before the age of 8 and 9 in girls and boys, respectively, due to early activation of the hypothalamic-pituitary-gonadal axis. If acceleration of bone maturation caused by elevated sex hormones levels is left untreated, bone age (BA) progresses faster than chronological age (CA), decreasing final adult height. Early puberty (EP) refers to the onset of secondary sex characteristics in girls and boys between 8–9 and 9–10 years of age, respectively. Rapid progression of puberty at this age can affect final height, and gonadotropin-releasing hormone (GnRH) agonist treatment is administered in such individuals in Korea.
CPP is treated through periodic administration of GnRH agonist, which continuously stimulates the pituitary gland to desensitize gonadotrophic cells and inhibit gonadotropin release, limiting the production of sex hormones [1]. Several studies have reported that preservation of final height is greater when CPP treatment is initiated early. Specifically for girls, initiating GnRH agonist treatment after the age of 8 has been found to be ineffective in preserving final height [2-6]. Thus, this study aimed to assess whether GnRH agonist treatment is effective in preserving final height in girls diagnosed with EP by comparing them with girls diagnosed with CPP.
Materials and methods
1. Subjects
Girls who were diagnosed with CPP or EP at Pusan National University Hospital between March 2013 and February 2021 and completed GnRH agonist treatment were enrolled in this study. Girls who had an organic cause of precocious puberty who also underwent growth hormone therapy and who had other chronic comorbidities were excluded. All patients were administered 90 µg/kg leuprolide acetate or triptorelin acetate once every 4 weeks until a CA of 11 years or a BA of 12–12.5 years.
CPP was diagnosed based on the onset of breast development before the age of 8, a greater BA than CA, and a GnRH stimulation test showing peak luteinizing hormone value of 5 IU/L or higher and increased more than two-fold from baseline. EP was diagnosed based on onset of breast development at 8–9 years of age, a greater BA than CA, and a GnRH stimulation test indicating puberty performed at 8–9 years of age as described above.
2. Methods
Clinical characteristics of age, sex, height, weight, BA, midparental height (MPH), and Tanner stage were examined at baseline before beginning treatment. Height and BA were examined again at treatment completion and at final follow-up when the growth rate was less than 2 cm/yr and menarche was confirmed.
BA was interpreted using the Greulich-Pyle method [7], and breast maturity was assessed based on Tanner stage. CA-based standard deviation score (SDS) for height, weight, and body mass index (BMI) were calculated according to the 2017 Korean National Growth Chart for children and adolescents enacted by the Korean Pediatric Society [8]. BA-based SDS for height (height SDS for BA) was calculated by substituting CA with BA on the growth chart. Predicted adult height (PAH) was calculated by substituting height SDS for BA with the height for girls at 18 years 11 months on the growth chart.
PAH was calculated at baseline, at treatment completion, and at final follow-up to assess changes after treatment and the difference in changes between the groups.
3. Statistical analysis
All results are presented as mean±standard deviation. To compare CPP and EP groups, continuous variables were analyzed using independent t-test or Wilcoxon rank-sum test, and categorical variables were analyzed using chi-square or Fisher exact test. Correlation analysis in the EP group was performed using the Pearson correlation coefficient. A P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
4. Ethical statement
This study was approved by the Institutional Review Board (IRB) of Pusan National University Hospital, Busan, Korea (IRB No. 2106-039-104).
Results
1. Clinical characteristics of patients
A total of 246 patients was included in this study, with 40 in the CPP group and 206 in the EP group. Table 1 shows the baseline clinical characteristics of patients in each group.
At baseline, the mean CA was 88.45±8.14 months in the CPP group and 103.18±3.60 months in the EP group, while the mean BA was 113.78±12.69 months in the CPP group and 125.29±8.98 months in the EP group, showing that BA progressed faster than CA in both groups. The gap between BA and CA was 25.33±11.01 and 22.11±7.98 months in the CPP and EP groups, respectively, and was not significantly different (P=0.085). At baseline, there were no significant differences in height SDS, PAH, and MPH between the 2 groups (P=0.427, P=0.173, and P=0.601, respectively).
At treatment completion, the mean CA was 130.45 months in the CPP group and 132.77 months in the EP group, while the mean BA was 144.43 months in the CPP group and 145.16 months in the EP group. The gap between BA and CA was 13.79±7.95 and 12.34±7.10 months in the CPP and EP groups, respectively, and was not significantly different (P=0.374). At treatment completion, there were no significant differences in height SDS and PAH between the 2 groups (P=0.865, P=0.180, respectively). The mean treatment duration was 42.83 months and 29.96 months in CPP and EP groups, respectively. The mean follow-up duration was 55.45 months and 39.21 months in CPP and EP groups, respectively.
2. Changes of PAH after GnRH agonist treatment
Table 2 shows PAH changes at treatment completion and final follow-up compared to baseline.
Among the 28 patients in the CPP group with BA tested after completion of treatment, the PAH significantly increased from 153.67±4.95 cm at baseline to 156.01±4.61 cm at treatment completion (P<0.001). The PAH significantly increased to 158.52±6.04 cm (P<0.001) among the 27 patients with BA tested at final follow-up. There was no significant difference between the MPH and the PAH at final follow-up (P=0.117).
We found similar results in the EP group. Among the 162 patients in the EP group with tested BA after treatment completion, the PAH significantly increased from 154.77±3.72 at baseline to 157.27±3.60 at treatment completion (P<0.001). The PAH significantly increased to 159.31±4.26 at final follow-up (P<0.001) among the 101 patients with BA tested at final follow-up.
Furthermore, we found no significant difference in the amount of PAH increase at completion of treatment compared to baseline between the CPP group and the EP group (P=0.358). Similarly, there was no significant difference in the amount of PAH increase at final follow-up compared to baseline between the CPP group and the EP group (P=0.480).
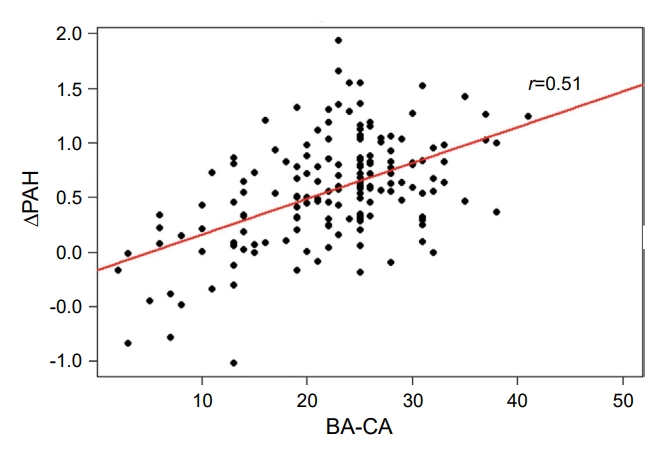
To determine whether other factors were related to changes in PAH (ΔPAH) in the EP group, correlation analysis with age at start of treatment, BA-CA, height SDS, weight SDS, BMI SDS, MPH, and treatment duration was performed in the EP group. ΔPAH showed positive correlations with age (r=0.06, P=0.470), BA-CA (r=0.51, P<0.001), height SDS (r=0.12, P=0.126), MPH (r=0.04, P=0.597), and treatment duration (r=0.07, P=0.350). On the other hand, ΔPAH exhibited a negative correlation with BMI SDS (r=-0.06, P=0.429). The only statistically significant correlation was between ΔPAH and BA-CA at the start of treatment (Table 3, Fig. 1).

The analysis of correlation between changes in PAH and clinical factors at the start of treatment in EP group
Discussion
This study compared PAH using height SDS for BA before and after GnRH agonist treatment between CPP and EP groups to assess whether this treatment is effective in preserving final height in girls with EP diagnosed after the age of 8. Compared to baseline, both groups experienced a significant increase in PAH after completion of GnRH agonist treatment as well as at final follow-up. Moreover, we found no significant difference in the amount of increase between the 2 groups.
The prevalence of precocious puberty is increasing in Korea. According to the 2019 Korea National Health and Nutrition Examination Survey published by the Health Insurance Review and Assessment Service, the incidence of female precocious puberty increased from 89.4 per 100,000 population in 2008 to 415.3 per 100,000 population in 2014. This survey also found 8–9 years to be the most common age at diagnosis (1,642.7 per 100,000), followed by 7–8 years (440.6 per 100,000) [9].
Precocious puberty not only decreases final adult height, but also provokes anxiety in patients and their families due to dramatic physical changes; such anxiety may impair academic performance or increase anti-social behaviors. Thus, the goal of treatment has been to minimize the loss of final height and reduce psychosocial problems by promoting timely puberty.
Periodic administration of GnRH agonists has been established as the standard treatment for CPP, and this treatment is also performed on girls aged 8–9 years with EP in Korea. However, several studies have reported that initiating treatment after the age of 8 years does not affect final height. Lazar et al. [10] reported that girls diagnosed with CPP before the age of 6 reached their predicted final height after GnRH agonist treatment. On the contrary, girls diagnosed between 6–8 years of age and girls who began development of secondary sex characteristics between 8–9 years of age did not reach their predicted final height even after GnRH agonist treatment. Similarly, Bouvattier et al. [11] reported that final adult height did not differ between GnRH agonist-treated and nontreatment groups in girls who began developing secondary sex characteristics between 8.4–10 years of age. In an assessment of girls diagnosed with CPP between the age of 7.5–8.5 years, Cassio et al. [12] reported that final height did not differ between the GnRH agonist-treated and nontreatment groups. Furthermore, a review article by Kletter and Kelch [13] reported that GnRH agonist treatment does not significantly alter final height in girls diagnosed with CPP after the age of 6 years. However, in this study, we found that GnRH agonist treatment increased the PAH in the EP group (diagnosed after age 8) similar to that in the CPP group. We observed a decrease in the BA progression rate compared to CA during treatment in both the CPP and EP groups, which might have an effect on the increase of PAH in the EP group. As a result of correlation analysis in the EP group, the greater was the progression of BA compared to CA at the start of treatment, the larger were the changes in PAH; there was no significant correlation with other factors.
Final adult height of children is estimated by the Bayley-Pinneau method [14] or Tanner-Whitehouse method [15] using CA, BA, and height. These methods were developed with reference to the Caucasian population and produce differing results from those using average adult height in the Korean population. Therefore, in this study, we estimated final adult height by first calculating height SDS corresponding to BA at baseline with reference to the 2017 Korean National Growth Chart for children and adolescents8) and then calculated height corresponding to this height SDS in females at 18 years 11 months of age, which was used as the near-adult height. Because there is no standard method for predicting adult height based on BA in the Korean population, we propose this method that utilized the growth chart.
One limitation of this study pertains to its retrospective nature, which could have hindered accurate comparisons because many patients did not undergo BA testing at points of height assessment. Additionally, although previous studies have reported that GnRH agonist treatment performed after the age of 6 is not effective in preserving final height, our contradicting results may have been affected by our inclusion of girls diagnosed before the age of 6 as well as girls between 6–8 years of age in the CPP group.
In conclusion, our study demonstrates that both CPP and EP patients had significantly increased PAH after GnRH agonist treatment, with no significant difference in the amount of increase between the 2 groups. This shows that GnRH agonist treatment can be helpful in preserving final height in patients diagnosed with EP after the age of 8 years as well as those diagnosed with CPP.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a clinical research grant from Pusan National University Hospital in 2022.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: MJK; Data curation: MJK, EHY; Formal analysis: MJK; Methodology: MJK; Project administration: MJK; Visualization: MJK; Writing - original draft: MJK, EHY; Writing - review & editing: MJK, EHY, HYJ, SJP, HWY, SHC, HYK, KHP, YMK
Acknowledgements
This work was supported by the Department of Biostatistics, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.