Tailored management of life-threatening complications related to severe obesity in a young adult with Prader-Willi syndrome
Article information
Abstract
Prader-Willi syndrome (PWS) is characterized by hypotonia, distinctive facial features, hyperphagia, obesity, short stature, hypogonadism, intellectual disability, and behavior problems. Uncontrolled hyperphagia can lead to dangerous food-seeking behavior and with life-threatening obesity. Severe obesity is prone to obstructive sleep apnea (OSA) and can lead to cor pulmonale. This study reports on a case involving a 21-year-old man with PWS who developed OSA due to severe obesity, which led to cor pulmonale, a life-threatening complication. Multidisciplinary care provided in the intensive care unit included weight reduction, ventilation support, antipsychotics, sedative drugs, rehabilitation, and meticulous skin care. The patient did recover. To prevent severe obesity in adults with PWS, hyperphagia must be controlled, and the patient must also be managed by an endocrinologist throughout childhood.
Highlights
· PWS patients with severe obesity are predisposed to have cor pulmonale, an alteration in the structure. To prevent severe obesity in adults with PWS, hyperphagia must be controlled through diet, and an endocrinologist must navigate care throughout childhood.
Introduction
Prader-Willi syndrome (PWS) is a multisystem genetic disorder caused by a lack of expression of paternal alleles in the PWS region of chromosome 15q11–13 [1]. It has an estimated prevalence of 1 in 10,000–30,000 live births, and the main clinical features are neonatal hypotonia, distinctive facial features, and poor growth in infancy followed by hyperphagia with severe obesity, short stature, hypogonadism, intellectual disability, and behavior problems [2]. Weight development in PWS is unique. Body weight is slightly reduced at birth but low or normal during the first 2 years of life due to poor feeding. This period is followed by rapid weight gain, resulting in a weight-for-height index that exceeds the normal range at the age of 10 years in nearly all patients with PWS [3,4].
Obesity-related complications include metabolic syndrome, type 2 diabetes mellitus (DM), septicemia due to skin infections, obstructive sleep apnea (OSA), and hypoventilation [5]. Morbid obesity often causes OSA, which can reduce oxygen levels in the blood, necessitating supportive devices to increase oxygenation during sleep [6]. PWS patients with severe obesity are predisposed to have cor pulmonale, an alteration in the structure (e.g., hypertrophy or dilatation) and function of the right ventricle (RV) of the heart caused by a primary disorder of the respiratory system resulting in pulmonary hypertension [7]. Therefore, severe obesity is the most serious and most common problem that contributes to morbidity and mortality in adults with PWS [2].
We describe a case of a 21-year-old male with PWS who presented with the life-threatening complication of cor pulmonale due to marked obesity and OSA whose condition improved with multidisciplinary management, including weight reduction through calorie restriction, diuretics, respiratory support, skin care, and rehabilitation.
Case report
The patient was born by cesarean section at the gestational age of 39 weeks with a birth weight of 3.3 kg (50th –75th). There was no family history of PWS. The patient did not cry at birth and demonstrated poor feeding; he was also diagnosed with bilateral undescended testicles. He became obese at age 4 and showed hyperphagia at age 5. At the age of 8 years old, he visited our hospital and was diagnosed with PWS by methylation polymerase chain reaction. His height and weight were 151 cm (1.06 standard deviation score [SDS]) and 75 kg (3.74 SDS), respectively. He started to receive recombinant growth hormone (rGH) treatment and methylphenidate for attention deficit hyperactivity disorder. Polysomnography revealed OSA, and he underwent a tonsillectomy at the age of 9.
At the age of 15 years old, a cocktail test revealed gonadotropin-releasing hormone deficiency and growth hormone deficiency. However, his parents did not want him to undergo sex hormone treatment. He presented with an uncontrolled glycemic status and osmotic symptoms (polyuria, polydipsia, and polyphagia). He was diagnosed with DM and therefore stopped the rGH therapy and began taking metformin. His body mass index (BMI) was 50.87 kg/m2 (weight, 134.5 kg; height, 162.6 cm). At the age of 19 years, he began taking glipizide and long-acting insulin (glargine). Because the patient's oxygen saturation decreased to 50% when he was sleeping, he was advised to apply positive pressure ventilation. However, the patient showed poor compliance. Two-dimensional echocardiography revealed a normal ventricle size and normal systolic function.
At 21 years of age, the patient presented with dyspnea and loss of consciousness. His height and weight were 164 cm and 185.7 kg, respectively (BMI, 69.04 kg/m2). His vitals were as follows: blood pressure, 96/25 mmHg; body temperature, 39℃; heart rate, 93 beats per minute; respiratory rate, 28 per minute; and oxygen saturation, 63%. According to his mother, he had gained 40 kg in one week and exhibited dyspnea, oliguria (<100 mL/day), and abdominal distension. On examination, we observed periorbicular cyanosis, respiratory distress, skin ulcers (1×3 cm in size on the left thigh [Fig. 1A] and 0.5×1 cm in size on the right lower arm), and a macular rash on his entire body. Chest x-ray showed cardiomegaly and haziness in the right lung (Fig. 2A). Arterial blood gas analysis revealed a pH of 6.9, PCO2 of 147, PO2 of 72.1, and HCO3 of 29.3. The patient was admitted to the intensive care unit (ICU) after intubation with synchronized intermittent mandatory ventilation (SIMV). Laboratory tests revealed a hemoglobin level of 15 g/dL (13.6–17.4 g/dL), a white blood cell level of 19,620/μL (3,800–10,580/μL), an erythrocyte sedimentation rat of 49 mm/hr (0–22 mm/hr), a C-reactive protein of 2.37 mg/dL (0–0.5 mg/dL), lactic acid of 10.37 mmol/L (0.5–2.2 mmol/L), albumin of 3.8 g/dL (3.5–5.2 g/dL), aspartate aminotransferase/alanine aminotransferase of 51 U/L (0–40 U/L)/59 U/L (0–41 U/L), blood urea nitrogen/creatinine of 34.9 mg/dL (6–23 mg/dL)/0.93 mg/dL (0.7–1.2 mg/dL), a fasting glucose level of 141 mg/dL, a glycosylated hemoglobin (HbA1c) of 8.2%, thyroid stimulating hormone/free T4 of 2.576 uIU/mL (0.55–4.78 uIU/mL)/1.01 ng/dL (0.89–1.8 ng/dL), a Troponin I level of 0.844 ng/mL (0–0.040 ng/mL), creatine kinase-MB of 3.73 ng/mL (0–5 ng/mL), and a pro-B-type natriuretic peptide (proBNP) of 1,904 pg/mL (0–88 pg/mL).
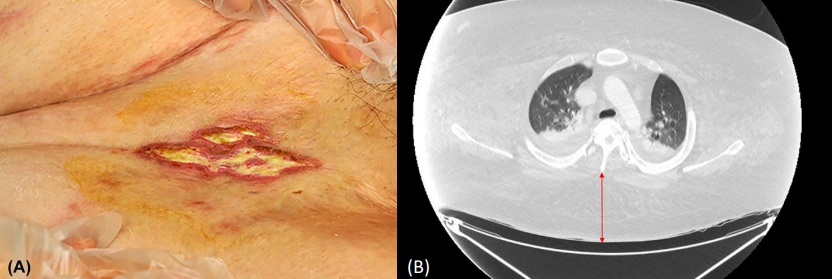
(A) A skin ulcer on the left thigh. The inguinal area was folded, and a 1×3-cm-sized ulcer demonstrated a yellowish discharge. (B) Chest contrast computed tomography (CT) at admission. The CT revealed bilateral pleural effusion and peribronchial opacity. The subcutaneous fat (arrow) under the skin on CT was 82.7mm.

(A) Changes in body weight and input/output balance. A continuous intravenous (IV) infusion of furosemide was used to achieve a negative balance of input/output (I/O) (negative 1,000–2,000 mL/day). The patient lost 54.3 kg (a reduction from 185.7 to 131.4 kg) over 7 weeks of hospitalization (HD). Serial chest x-rays showed cardiomegaly with combined pulmonary congestion and pleural effusion on HD 4, subsegmental atelectasis in the right middle lung field on HD 28, and improved pulmonary congestion and pleural effusion on HD 42. (B) Caloric restriction and glucose control. Total parenteral nutrition (TPN) was begun on HD 4. On HD 14, enteral nutrition was started via a nasogastric tube at 100 mL/day and was increased to 1,500 mL/day over 4 weeks. To restrict calories (1,500 kcal/day), TPN was decreased according to the increasing enteral feeding volume. As the amount of enteral feeding increased, the patient's blood glucose level rose, and insulin injections were required beginning on HD 28. On HD 35, we switched to full enteral feeding via a nasogastric (NG) tube. On HD 42, we began oral feeding (1,000 kcal/day). At discharge, an insulin dosage of 0.83 IU/kg/day (degludec at 50 IU/day and aspart at 59 IU/day) was required.
Chest computed tomography revealed bilateral pleural effusion and peribronchial opacity (Fig. 1B). We provided normal saline and noradrenalin for hemodynamic restoration, cefotaxime for septic shock, midazolam and fentanyl for sedation, and physiotherapy. Testing for any respiratory virus was negative. Transesophageal echocardiography revealed a normal-sized left ventricle and normal systolic function; however, we noted a dilated RV cavity, decreased RV systolic function, and pulmonary hypertension (RV pressure of 52 mmHg), which suggested right heart failure (RHF). Cor pulmonale was diagnosed. We started a continuous infusion (3 mg/hr) of furosemide to reach a negative balance of input/output (I/O) (negative 1,000–1,500 mL/day) and caloric restriction (1,500 kcal/day). The patient was placed in a special ICU bed with a pressure-relieving mattress and a weighing machine. His positioning was changed every 2 hours for skin care from the first day of hospitalization (HD). We added clindamycin and dressed his wounds daily. On HD 4, we began total parenteral nutrition because of a large volume of gastric regurgitation.
On HD 7, the ventilator mode was changed from volume control (VC) SIMV to pressure-control (PC) SIMV due to repeated bronchoconstriction. By HD 10, the patient had lost 5.8 kg (3.1%) (Fig. 2A). However, pulmonary congestion and pleural effusion did not improve; therefore, the target balance of I/O was adjusted to -1,500–2,000 mL/day. The patient lost 1.8 kg per day, and his lung congestion improved (Fig. 2A). On HD 14, we started enteral nutrition via a nasogastric tube at a rate of 100 mL/day to 1,500 mL/day over 4 weeks (goal: 1,500 kcal/day, 1,500 mL/day, and 70 g/day of protein) (Fig. 2B). Fluids and electrolytes were corrected daily according to body weight. On HD 28, the patient's weight was 150.6 kg (-35.1 kg), and he was successfully extubated. Oxygen was delivered at a high flow rate of 50 L/min, and the fraction of inspired oxygen (FiO2) was 0.4 via a nasal cannula. As the patient's lung congestion gradually improved, the high flow rate was decreased to 20 L/min.
By HD 35, the high flow was turned off, and biphasic positive airway pressure (BiPAP) was applied only during sleep. The patient was able to receive rehabilitation training while awake to retrain his body to perform basic movements. From this point on, the skin ulcers healed, and any dry crust was removed. On HD 36, the patient was transferred to the general ward, and his weight had decreased to 133 kg (-52.7 kg). On HD 42, oral feeding (1,000 kcal/day) began, and the patient was discharged at a weight of 131.4 kg (-54.3 kg) on HD 49. Before discharge, we educated the patient and his parents regarding drug adherence, diet, oxygen therapy with BiPAP only during sleep, and exercise training for muscle stretching and an active range of motion. He was discharged on the following medications: furosemide at 0.3 mg/kg/day, spironolactone at 0.19 mg/kg/day, metformin at 15.2 mg/kg/day, and insulin at 0.83 IU/kg/day (degludec at 50 IU/day and aspart at 59 IU/day). For 3 years after discharge, the patient has visited the outpatient clinic every 3 months, and he has not been hospitalized. His HbA1c level has been maintained at 8%–9% with metformin (13.3 mg/kg/day) and insulin (degludec at 76 IU/day and aspart at 60 IU/day). He has been continuing oxygen therapy with BiPAP during sleep without congestive hepatopathy or elevation of the proBNP level.
Discussion
This report details a young adult with PWS who suffered from cor pulmonale due to severe obesity. This patient had several obesity-related health problems, including type 2 DM, dyslipidemia, skin ulcers, and OSA. OSA should be suspected when a patient shows excessive daytime sleepiness associated with chronic heavy nocturnal snoring. During sleep, pulmonary arterial pressure is elevated due to an increase in pulmonary resistance resulting from hypoxic vasoconstriction [8]. Daytime hypoxemia also plays a major role in permanent daytime pulmonary arterial hypertension and RHF, as was the case for this patient. Cor pulmonale may develop if this is not reversed [9]. Rapid weight gain, swelling of the ankles, marked shortness of breath, cyanosis, and decreased activity are signs of cardiac decompensation [6]. Our patient had all these symptoms when he presented to the emergency department. Unrecognized RHF can contribute to pneumonia, reactive airway disease, and sudden death in obese patients with PWS [10]. RHF treatment involves diuretics (most often furosemide) and oxygen therapy [11]. We did not use digitalis because our patient did not show left heart failure or arrhythmia.
Our patient was admitted to the ICU and received respiratory support, I/O control, calorie restriction, skin care, rehabilitation training, and nursing assistance. Although his ideal body weight for his height was 60.5 kg, we targeted a 45-kg body weight reduction because he had not experienced any respiratory problems when his body weight was 140 kg 2 years earlier. According to the Harris-Benedict equation [12], the basal energy expenditure of a 150-cm tall, 19-year-old male at 100 kg and 47.8 kg should be 3,244 and 1,566 kcal, respectively. Therefore, we set a calorie intake of 1,500 kcal/day for this patient. Extreme caution was used to assure a safe reduction in body weight using laboratory tests and daily body weight measurements. Enteral feeding was initiated according to the relevant guidelines. [13].
In the early phase of the patient's ICU stay, ventilation support for very high airway pressure was a problem in the full sedative state. During mechanical ventilation, the airway pressure tended to be very high due to low respiratory system compliance associated with morbid obesity and repeated bronchoconstriction [14]. On day 7 of mechanical ventilation, the ventilatory mode was changed from VC to PC due to repeated bronchoconstriction. After that, the patient's bronchoconstriction improved.
The patient's severe obesity and accompanying behavioral problems made ICU care difficult. At least 4 nursing staff members were required to control his agitation and irritability, including biting the endotracheal tube, hitting a bed rail, and self-extubation. A sedative drug should be tapered to wean a patient from a ventilator. However, we could not reduce the amount of sedative drugs due to the patient's agitation and irritability. To control aggressive behavior without a sedative effect, we applied aripiprazole (0.1 mg/kg/day). When the patient was aggressive towards the medical staff, we administered a haloperidol (2.5 mg) intramuscular injection. The patient was extubated on HD 28. Collaboration between the pulmonologist, cardiologist, psychiatrist, nurse, nutritionist, and endocrinologist is typically required to manage a severely obese adult patient with PWS.
Research [1] on the causes of death in PWS patients reports that respiratory failure (73%) is the main cause in those under 3 years of age. Respiratory failure (49%), choking (12%), and neurologic conditions (12%) are the main causes of death in patients aged 3–12 years. Respiratory failure (25%), cardiac incidents (15%), accidents (15%), and infection (15%) are responsible for death in those aged 12–18 years old. In patients over the age of 18, respiratory failure (26%), cardiac problems (19%), gastrointestinal issues (10%), infection (10%), and obesity (9%) were noted as causes of death. The annual mortality rate of adults with PWS is approximately 3%, and the average age at death is 33 years [1]. Patients with PWS are being diagnosed at an earlier age, especially during the neonatal period, which allows for the application of early interventions, including rGH therapy and diet control. However, a family should pay attention to the patient's food access during daily life. A comprehensive multidisciplinary approach is needed to avoid obesity through the introduction of a low-calorie and well-balanced diet together with appropriate psychological and behavioral counselling for the family. Controlling food access does not manage the preoccupation with food-related behaviors in PWS [2]. Regular recreational activities are also recommended to distract the patient from the search for food. Rigorous supervision along with restriction of access to food and money and regular exercise can sometimes achieve adequate weight control. However, this approach is ineffective in older PWS patients that were diagnosed after becoming obese. Currently, several clinical trials (e.g., GLP-1 agonists, oxytocin and analogs, diazoxide, and unacylated ghrelin etc.) in PWS patients are ongoing [15]. Above all, each patient requires an individualized approach.
In conclusion, we report on the successful treatment of life-threatening cor pulmonale due to morbid obesity in a young adult patient with PWS. The multidisciplinary care offered included weight reduction through calorie restriction and diuretics, respiratory support, skin care, and rehabilitation in the ICU. To prevent severe obesity in adults with PWS, hyperphagia must be controlled through diet, and an endocrinologist must navigate care throughout childhood.
Ethical statement
We obtained informed consent from the patient's parents.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a grant from Samsung Medical Center (GFO3200061#).
Author contribution
Conceptualization: MK, SYC; Data curation: MK; Formal analysis: MK; Methodology: MK, JK; Visualization: MK; Writing - original draft: MK, SYC, DKJ; Writing - review & editing: MK, SYC, DKJ
