Immobilization-induced symptomatic hypercalcemia treated with zoledronate in a child with a left ventricular assist device
Article information
Abstract
Differential diagnosis of hypercalcemia in children includes confirmation of hyperthyroidism, infection, inflammatory processes, and malignant tumors. Immobilization-induced hypercalcemia is rare in healthy individuals, although it can occur in adolescent males, especially after fracture. Immobility can cause increased skeletal calcium release and hypercalcemia, and this condition is also known as resorptive hypercalcemia. We present a case of a 10-year-old adolescent girl with advanced heart failure who underwent implantation with a HeartMate 3 left ventricular assist device. She had symptoms of abdominal pain, vomiting, and constipation on the fifth month of hospitalization. She subsequently developed immobilization-induced symptomatic hypercalcemia (serum calcium, 12.1 mg/dL; corrected calcium 12.8 mg/dL; parathormone, 1.9 pg/mL; calcium/creatinine ratio in spot urine, 1.21). However, hypercalcemia is uncommon in children with advanced heart failure. Bisphosphonate therapy was initiated because our patient did not respond to hydration and furosemide treatment, and she had persistent abdominal pain, vomiting, and constipation. The patient's complaints were resolved on the second day after administrating bisphosphonate, and hypercalcemia did not recur.
Highlights
· Immobility can be caused by increased skeletal Ca+ release and hypercalcemia. Hypercalcemia caused by immobilization is rare in prolonged critical illness processes within PICU patients.
· Bisphosphonate is indicated if there is no response to hydration and loop diuretics treatments.
Introduction
Immobilization-induced hypercalcemia is common in individuals with rapid bone turnover, such as spinal cord injuries, fractures, or Paget's disease [1]. It occurs when osteoclastic bone resorption exceeds the rate of osteoblastic bone formation, causing an imbalance in the bone remodeling process [2]. Such hypercalcemia is either asymptomatic or has vague clinical features [3]. However, it is occasionally severe and can cause various symptoms, such as neuromuscular findings, seizures, and respiratory arrest [3]. Albright was the first to describe immobilization-induced hypercalcemia in 1941, in a 14-year-old adolescent [4]. In that case report, the patient had developed hypercalcemia and hypercalciuria after immobilization secondary to a femoral neck fracture and had undergone a series of investigations and treatments, including parathyroidectomy, before the correct diagnosis was made [3].
Children with advanced he art failure have risk for immobilization even after left ventricular assist device (LVAD) implantation and can result in a prolonged hospital stay and debilitating complications such as respiratory muscle weakness and neurologic sequelae [5,6]. The use of LVAD as a bridge for heart transplantation in the pediatric population has increased rapidly [7]. We present here an adolescent girl implanted with a HeartMate 3 (HM3; Abbott Corporation, Abbott Park, IL, USA) LVAD who developed symptomatic immobilization-induced hypercalcemia that was successfully treated with fluid and bisphosphonate (zoledronate).
Case report
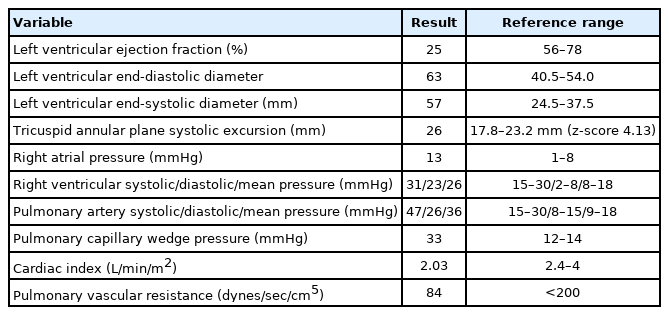
A 10-year-9-month-old girl was admitted on the diagnosis of dilated cardiomyopathy. Her height was 162 cm and weight was 50 kg (body surface area, 1.51 m2). Chest x-ray showed cardiomegaly and pulmonary edema. Transthoracic echocardiography revealed a dilated left ventricle and moderate mitral and tricuspid regurgitation. Left ventricular ejection fraction was 25%, left ventricular end-diastolic diameter was 63 mm, and left ventricular end-systolic diameter was 57 mm. Tricuspid annular plane systolic excursion was 26 mm (Table 1). Her initial treatment had consisted of inotropic support with milrinone, furosemid, and fluid restriction (<1,000 mL/m2).
Laboratory results showed preserved renal function and elevated liver enzymes: serum creatinine, 0.82 mg/dL; blood urea nitrogen, 22 mg/dL; aspartate aminotransferase, 385 IU/l; alanine aminotransferase, 230 IU/l; total bilirubin, 1.44 mg/dL; serum calcium (Ca), 9.3 mg/dL (normal range: 8.8–10.6); phosphorus, 3.2 mg/dL; lactate, 2.2 mg/dL; and elevated brain natriuretic peptide, 15,468 ng/L (Table 2). On the 13th day of admission, her general condition gradually worsened and required accelerated doses of epinephrine. Right heart catheterization revealed elevated right atrial (13 mmHg), right ventricular (systolic/diastolic/mean, 31/23/26 mmHg), and pulmonary artery pressures (systolic/diastolic/mean, 47/26/36 mmHg) and a pulmonary capillary wedge pressure of 33 mmHg. Her cardiac index was 2.03 L/min/m2, and pulmonary vascular resistance was 84 dynes/sec/cm+ (1 Wood unit). Cardiac enzymes and thyroid function tests were normal.
Despite maximal inotropic support, the patient's condition deteriorated. After a multidisciplinar y transplant team discussion, she underwent centrifugal continuousflow HeartMate 3 LVAD implantation for bridge-to-heart transplantation on her 20th day of pediatric intensive care unit (PICU) admission. After LVAD implantation, her inotropic support and inhaled nitric oxide volumes were decreased. LVAD settings were adjusted according to the patient's daily echocardiographic findings. LVAD pump-flows were maintained between 3.2–3.8 L/min.
The anticoagulation protocol consisted of heparin infusion for a goal partial thromboplastin time of 1.5–2 times the normal value, which was initiated on postoperative day 2, followed by low molecular-weight heparin. Subsequently, oral anticoagulant (warfarin) was initiated to target the international normalized ratio goal of 2–3 after hepatic and renal function recovery. Antiplatelet therapy of aspirin (1 mg/kg/day) was started following recovery of platelet count. She received additional oral medications including enalapril, sildenafil, and spironolactone. She required both invasive and noninvasive respiratory support during her PICU stay and eventually required tracheostomy for active bronchial aspirations on the 47th day of admission.
The patient was immobile and suffered from severe muscle weakness during her PICU stay. We consulted pediatric neurology and confirmed that the patient could be experiencing critical illness polyneuropathy. Further, because the patient typically laid in bed for the entire day, she was not performing exercises recommended by physiotherapists and required long-term support from the mechanical ventilator. She had open wounds due to immobilization in the sacral region, but no growth occurred in culture from the wound. Our patient had a stool frequency of one stool/wk. Lactulose treatment was started due to constipation. After the patient received the LVAD implant, her food intake decreased compared to levels prior to the operation. The patient's weight was 50 kg at the time of hospitalization but dropped to 45 kg after 3 months. The pediatric psychiatry team was consulted, after which she began receiving sertraline treatment.
Severe abdominal pain and vomiting developed in the fifth month of hospitalization. Abdominal examination revealed no evidence of defense or rebound. Abdominal ultrasonography was normal except for a 1-mm stone observed in the right kidney. The pediatric nephrology group was consulted and determined that the stone that did not cause obstruction in the right kidney and could not explain her current clinical status.
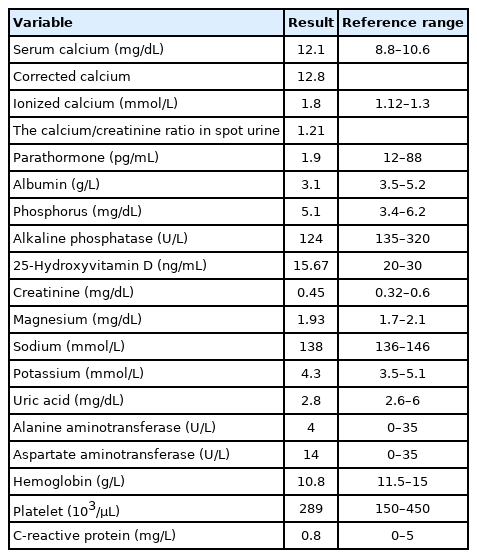
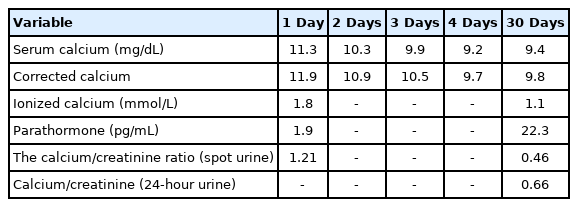
Chronic kidney disease was not considered within the 1–2 months before symptom onset, as her renal function tests and glomerular filtration rate were normal. The patient was given fluid therapy that involved 5% dextrose and 0.9% NaCl administration at the same time interval. The patient was fed enterally through the oral route with hospital meals that contained acceptable levels of calcium. In addition, the patient was not given supplemental calcium therapy during this period. However, her serum calcium increased to 12.1 mg/dL and corrected Ca increased to 12.8 mg/dL during her fifth month of hospitalization. The complete urine analysis and culture were normal, but urinary calcium excretion increased. The calcium/creatinine ratio in the spot urine was 1:1.21. Other laboratory results showed preserved renal and liver function. Her serum phosphorus level was 5.1 mg/dL, parathormone (PTH), 1.9 pg/mL (normal range, 12–88 pg/mL), and 25-hydroxyvitamin D was 15.67 ng/mL (normal range, 20–30 pg/mL) (Table 3). PTH was measured by the whole-PTH method (Beckmann Coulter Inc., Fullerton, CA, USA). During this period when the patient was hypercalcemic, she did not take any medications that could cause hypercalcemia, such as thiazide, lithium, and theophylline. In addition, no conditions that could cause hypercalcemia such as infection, fracture, and mechanical problems were detected. Because of the hypercalcemia, her fluid therapy was increased to 2,000 mL/m2, her oral feeding was stopped, and furosemide was started at 1 mg/kg/day. Abdominal symptoms persisted for 10 days. Based on these symptoms, the multidisciplinary team considered immobilization-induced hypercalcemia and commenced zoledronate therapy. Her calcium level decreased to 9.2 mg/dL on the second day after zoledronate therapy (2.5 mg [0.05 mg/kg]), and her alkaline phosphatase was 115 U/L, ionized Ca was 1.1 mmol/L, and PTH was 22.3 pg/mL. Spot urine assessment indicated that the calcium/creatinine ratio was 0.46 (calcium, 15.98; creatinine, 34.3) (Table 3). The calcium/creatinine ratio was 0.66 in 24-hour urine. The patient’s abdominal pain and constipation resolved on the second day after zoledronate administration at the fifth month of hospitalization. Hypercalcemia did not recur. Nutritional support of the patient was increased, and fluid therapy and furosemide therapy were discontinued. Our patient was transferred to the pediatric cardiology service with a good clinical status and normal calcium value in the eighth month of PICU admission.
Discussion
We report a case of a patient who developed hypercalcemia caused by immobilization that persisted due to heart disease. Although this is rare among patients who can move freely, immobilization can be a cause of hypercalcemia among patients in the intensive care unit. Differential diagnosis of hypercalcemia includes hyperthyroidism, infection, inflammatory processes, prolonged immobilization (>2 weeks), or malignant tumors [1,3,8]. Immobilization-induced hypercalcemia is rare in healthy individuals, although it can occur in adolescent males, especially after a fracture [1]. Excessive reduction in mechanical stress on the bone reduces osteoblast-mediated bone formation and accelerates osteoclast-mediated bone resorption [8,9]. Further, imbalance between bone formation and resorption can result in severe hypercalcemia. Immobility can cause increased skeletal calcium release and hypercalcemia, and this condition is also known as resorptive hypercalcemia [8].
A diagnosis of hypercalcemia often is made incidentally when a high level of calcium is detected in the blood [3]. The classic definition of hypercalcemia due to immobilization of a patient without a pre-existing metabolic bone disease was established by Albright et al. [10] Albright et al. [10] also reported that the skeletal structure was exposed to many stresses, as well as hormonal and nutritional effects during normal activity. Excessive reduction in mechanical stress on the bone reduces osteoblast-mediated bone formation and accelerates osteoclast-mediated bone absorption [8,9]. During this separation of bone formation and resorption, a minority of patients develop severe hypercalcemia, which can affect renal function [9]. Immobility can be caused by increased skeletal Ca+ release and hypercalcemia [8].
Appropriate patient and device selection and timing, are critical for successful outcomes in pediatric patients with advanced heart failure. In this case, a multidisciplinary decision for LVAD implantation was determined before endorgan dysfunction but during escalating inotropic support. The U.S. Food and Drug Administration approved the HM3 device for advanced heart failure therapy in patients with a body surface area (BSA) >1.5 m2 either as a bridge-to-heart transplantation or as destination therapy. The reported low rate of thromboembolic and hemorrhagic complications with a HM3 device prompted us to pursue this alternative even though the patient's BSA was 1.36 m2 [5,11].
Our patient underwent complex surgery and LVAD implantation due to dilated cardiomyopathy and was immobile for a 5-month PICU hospitalization. LVAD use can be associated with complications such as infection, kidney failure, mechanical dysfunction, hemolysis, bleeding, arteriovenous fistula, arterial dissection, arterial laceration, and thrombosis [11]. The hospital stay of patients with LVAD is long, and these patients also have complications related to immobilization due to long-term hospitalization [5,11]. The mortality rate is high in these patients during the hospital stay [11]. In this case, a sacral wound and hypercalcemia developed due to immobilization.
Hypercalcemia complications can include constipation, weight loss, polyuria, polydipsia, reduced level of consciousness, impaired appetite, nephrogenic diabetes insipidus, reduced glomerular filtration rate, headache, and convulsion [3,8]. These complications can occur with acute immobilization for longer than 2–8 weeks [3,8]. Early treatment of hypercalcemia includes hydration and loop diuretics [4]. Since hypercalcemia causes dehydration, the first step of treatment should be hydration. Loop diuretics are used to increase calcium excretion from the kidneys [8]. Bisphosphonate is indicated if there is no response to hydration and loop diuretics treatments [4]. Zoledronate, which is a bisphosphonate, rapidly decreases bone resorption to regress hypercalcemia [12]. Bisphosphonate therapy was initiated because our patient did not respond to hydration and furosemide treatment, and she also experienced abdominal pain, vomiting, and constipation.
The hypercalcemia in our patient regressed on the second day after a low dose (0.05 mg/kg) of zoledronate therapy. Moreover, the abdominal pain and constipation complaints resolved on the second day after administrating zoledronate. After bisphosphonate therapy, the hypercalcemia regressed and did not recur, and no complications of hypocalcemia were observed. Zoledronate is a safe and useful treatment option for acute and transient hypercalcemia in hemodialysis patients [12]. In severe cases of hypercalcemia, hemodialysis with low calcium dialysate might be required [8].
In conclusion, hypercalcemia caused by immobilization is rare in prolonged critical illness processes within PICU patients. Immobilization-related symptoms such as muscle weakness, mechanical ventilation dependency, health care-associated infections, and decubitus ulcers are detected frequently. Nevertheless, immobilization-induced hypercalcemia and its related symptoms or signs can be challenging to assess and diagnose. Therefore, immobilization-induced hypercalcemia should be considered in prolonged PICU admissions that report severe gastrointestinal complaints; further, bisphosphonates should be considered in hypercalcemia treatment when refractory to standard therapies.
Notes
Ethical statement
Written informed consent was obtained from patients who participated in this study.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.