Current status of continuous glucose monitoring among Korean children and adolescents with type 1 diabetes mellitus
Article information
Abstract
Type 1 diabetes mellitus (T1DM) requires life-long insulin therapy because of diminished insulin-secretion capability. Glycemic control and glucose monitoring are important to prevent T1DM complications. Continuous glucose monitoring (CGM) measures glucose level, every one to five minutes, in the interstitial fluid from a subcutaneous sensor and facilitates better glycemic control, reduces hypoglycemia, and is safely used in the pediatric population. CGM can be categorized as retrospective, real-time, or intermittently scanned CGM, and all forms are available in Korea. The CGM device has 3 components: sensor, transmitter, and monitor/receiver. Key metrics of CGM include days of CGM application, percentage of time with CGM, mean glucose, glucose management indicator, glycemic variability, and use of Ambulatory Glucose Profile for CGM reports. CGM sensors and transmitters have been partly reimbursed by the Korean National Health Insurance Service (NHIS) since 2019, and 1,434 T1DM patients (male, 40.8%; age <20 years, 52.4%) in Korea were prescribed CGM as of December 2019. In Korea, the number of CGM users will increase due to reimbursement for CGM sensors and transmitters by the NHIS. Successful CGM use requires long-term policies to establish diabetes education and financial assistance. Clinicians should become well-acquainted with interpretation of CGM data and information updates to facilitate integration of CGM data into clinical practice among pediatric T1DM patients.
Introduction
Type 1 diabetes mellitus (T1DM) is caused by progressive loss of pancreatic beta cells as a consequence of autoimmune destruction that eventually leads to decreased insulin secretion [1,2]. Therefore, life-long insulin therapy is required in patients with T1DM to achieve optimal glycemic control and prevent acute and chronic complications [3]. Recent reports have shown an increasing incidence of T1DM worldwide as well as in Korea, and this might increase the associated burden of disease [4,5].
Self-monitoring of blood glucose (SMBG) and measurement of glycosylated hemoglobin (HbA1c) has been part of the standard care for diabetes management and is considered essential for glycemic control. Frequent measurement (6–10 times per day) of SMBG is associated with decreased HbA1c value [6-8]. SMBG shows blood glucose level at the time of testing but has several disadvantages such as need for a finger-prick and no information on trends between SMBG measurements. HbA1c reflects the average blood glucose level over approximately 3 months. Most of the recent guidelines recommend HbA1c <7.0% in children and adolescents with T1DM [8,9]. HbA1c has advantages such as non-fasting-sample-based measurement, being a reference marker for metabolic control, and strong association with diabetes-related complications [10,11]. However, limitations of HbA1c include its inability to reflect daily glycemic variability and the influence of hemoglobin concentration [12,13].
Continuous glucose monitoring (CGM) is a device-based testing method that circumvents the limitations of SMBG and HbA1c. CGM measures glucose level in the interstitial fluid every 1 to 5 minutes from a sensor inserted into subcutaneous tissue [14]. Technological advances in CGM devices have facilitated smaller size, affordable price, and increased accuracy. Several studies have shown CGM improves glycemic control and decreases hypoglycemia. Recent guidelines recommend CGM as an essential consideration for management of T1DM in children and adolescents [15-17]. In Korea, CGM sensors and transmitters have been partly reimbursed by the Korean National Health Insurance Service (NHIS) since 2019 and 2020, respectively. This review summarizes the current status of CGM among Korean children and adolescents with T1DM.
Types and components of the CGM system
There are 3 main types of CGM systems: retrospective (professional) CGM, real-time (personal) CGM, and intermittently scanned CGM (isCGM, or flash glucose monitoring). Retrospective CGM measures glucose continuously, blinded to patient. After 10 to 14 days of CGM, the data are analyzed in a clinic by healthcare providers. Real-time CGM measures glucose value continuously; unblinded to patient; and provides real-time glucose level, alarms for preset threshold, hypoglycemia and hyperglycemia, as well as glucose trends and predictions. Recently developed CGM systems have the capacity to transmit data to cloud storage, which enables sharing and tracing of CGM data among caregivers and healthcare providers. The isCGM measures glucose continuously but only shows glucose level at the time of sensor scan. In Korea, as of September 2020, 3 real-time CGM and 1 isCGM systems are available: the GuardianTM Connect CGM system (Medtronic Inc., Minneapolis, MN, USA), the Dexcom G5Ⓡ Mobile CGM system (Dexcom Inc, San Diego, CA, USA), and the FreeStyleⓇ Libre (Abbott Diabetes Care Limited, Witney, Oxfordshire, UK).
The CGM system has 3 components: sensor, transmitter, and monitor (receiver). The sensors are inserted into subcutaneous tissue and measure glucose level in the interstitial fluid for 6 to 14 days, depending on manufacturer. Most CGM sensors need to be calibrated at least twice daily, although isCGM sensors do not need recalibration as they are factory calibrated. Furthermore, "adjunctive" CGM devices require SMBG confirmation for therapeutic decision making, whereas "nonadjunctive" devices do not. Transmitters send the glucose measurement data to a monitor or receiver and may be functional for 3 months or 1 year based on the manufacturer. However, the isCGM does not have a transmitter and, instead, utilizes a monitor/receiver to receive and display data from a transmitter, such as a smartphone, smartwatch, or company-manufactured display terminal.
Key metrics for CGM
The key metrics for CGM data analysis were suggested in the Advanced Technologies & Treatments for Diabetes (ATTD) consensus recommendations of 2017 [15]. The recent ATTD consensus recommendations of 2019 suggested the use of standardized CGM metrics for clinical care [18]. Ten core metrics and recommendations for T1DM patients were selected: (1) number of days of CGM application (14 days) [19], (2) percentage of time with CGM (at least 70% of data from 14 days), (3) mean glucose, (4) glucose management indicator (formerly, estimated HbA1c) [20], (5) glycemic variability as % of coefficient of variation (≤36%), (6) time above range as % of readings and time >250 mg/dL (<5%), (7) time above range as % of readings and time 181–250 mg/dL (<25%), (8) time in range as % of readings and time 70–180 mg/dL (>70%), (9) time below range as % of readings and time 54–69 mg/dL (<4%), (10) time below range as % of readings and time <54 mg/dL (<1%), and use of Ambulatory Glucose Profile (AGP) for CGM reports.
The AGP report is a standardized, single-page report of CGM [21]. The updated version 4.0 of the AGP report displays 10 key metrics for CGM in "Glucose Statistics and Targets" and "Time in Range" sections (Fig. 1). Moreover, a graph presents the median along with 5th, 25th, 75th, and 95th percentile lines and target range (70–180 mg/dL) of the glucose profile over a 24-hour period, which summarizes glucose values from the reported period. In addition, daily glucose profiles are displayed.
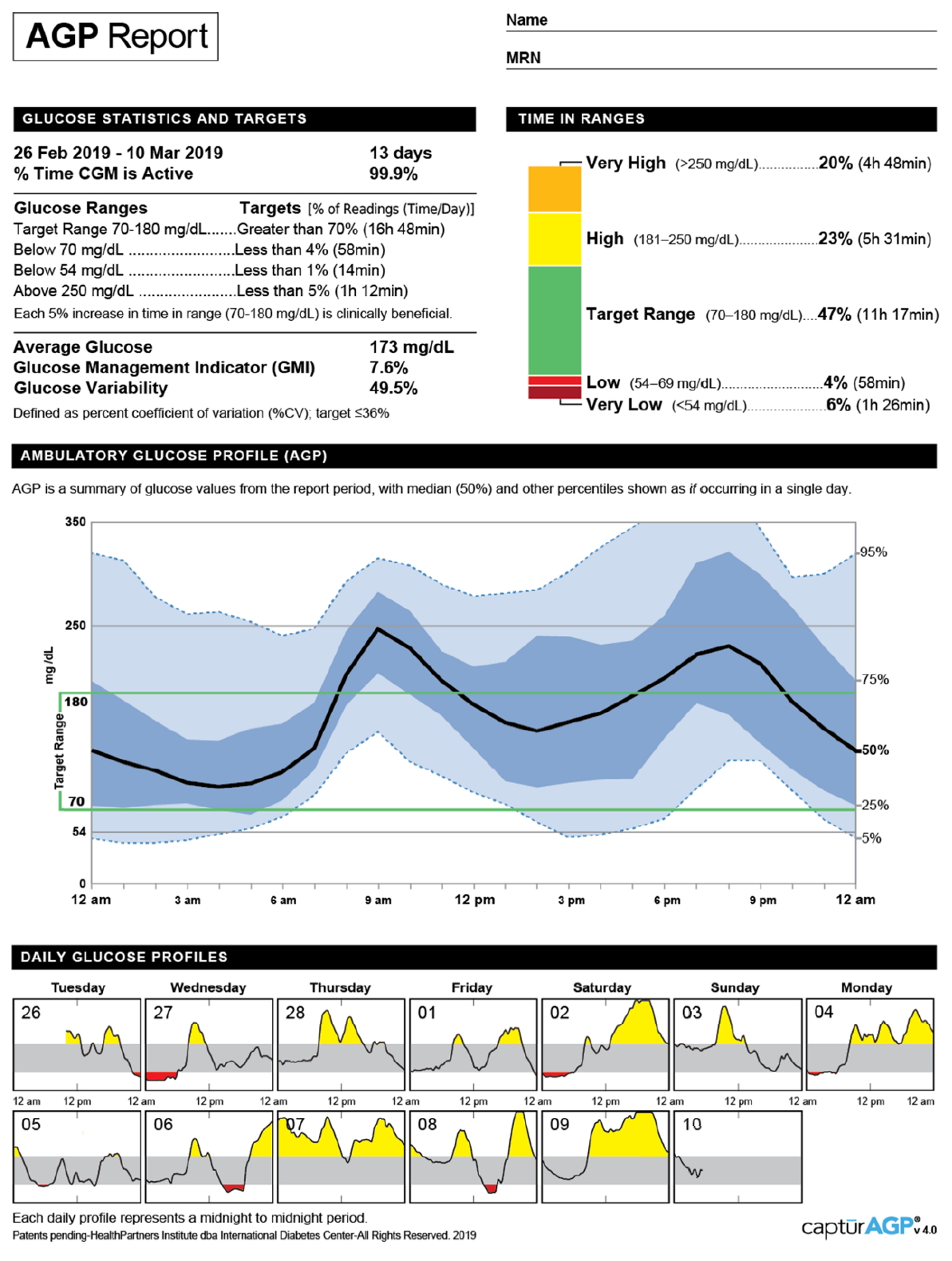
Ambulatory glucose profile report (v4.0) for continuous glucose monitoring by the International Diabetes Center (Available at: http://www.agpreport.org/agp/sites/default/files/2_About_CGM_AGP_V4.PNG). Adapted from International Diabetes Center for publication with permission.
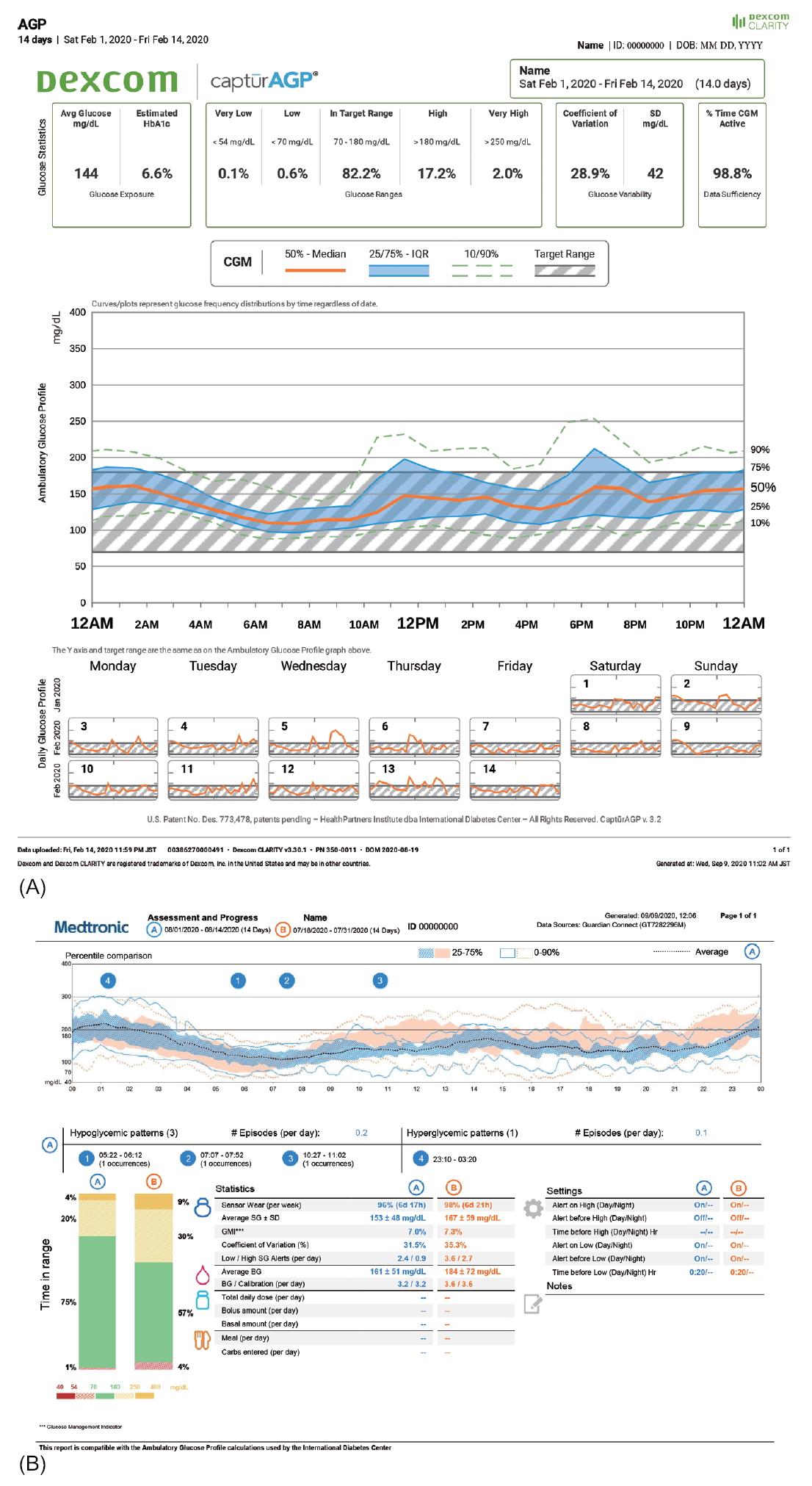
In Korea, the software packages available for CGM data analysis are CareLinkTM for GuardianTM Connect CGM system, Dexcom CLARITYⓇ for Dexcom G5Ⓡ Mobile CGM system and Dexcom G6Ⓡ CGM system, and LibreViewⓇ for FreeStyleⓇ Libre. Using these software packages, key metrics of CGM can be analyzed and reported (Fig. 2).
Efficacy of CGM
Several studies have reported that glycemic control is improved in pediatric T1DM patients who use CGM. In a study from the Juvenile Diabetes Research Foundation on CGM use, HbA1c decreased by 0.5% in adults but showed no significant difference in participants younger than 25 years due to poor adherence to CGM [22]. However, secondary analysis in a younger age group showed improved glycemic control with ≥6 days/wk of adherence [23]. Moreover, CGM was beneficial in maintaining good glycemic control and decreasing hypoglycemia in individuals with HbA1c <7.0% [24]. Further, CGM was effective in reducing HbA1c and glycemic variability, especially in participants who availed nearly daily application [25,26]. In a study among T1DM patients younger than 4 years, caregivers showed high satisfaction for CGM use with regard to hypoglycemia and effect on habits influencing glycemia and insulin-dose adjustment, although glycemic control was unsatisfactory [27].
In adult patients with T1DM, the use of CGM is associated with decreased episodes of hypoglycemia, especially in high-risk populations with hypoglycemia unawareness and/or severe hypoglycemic event [28-30]. In pediatric T1DM patients, several studies have shown decreased time spent in hypoglycemia and hypoglycemic episodes [24,31], although some studies did not report this benefit [22,32]. CGM might be helpful for high-risk pediatric patients with T1DM similarly as in adults. Further studies with updated CGM systems are needed in children and adolescents with T1DM.
Current status of CGM in Korea
In Korea, 3 types of real-time CGM, retrospective CGM and isCGM, are approved by the Ministry of Food and Drug Safety of Korea. The characteristics of CGM systems are summarized in Table 1.
Sensors and transmitters (and insulin pumps) of CGM systems have been reimbursed partially by the NHIS since January 2019 and January 2020, respectively. Approximately 40%–50% of the total cost of sensors and transmitters for CGM are paid by patients or caregivers. Following approval by the regulatory agencies in 2018, isCGM will become commercially available from May 2020. However, retrospective CGM is not covered by the NHIS of Korea.
According to the claim data of the NHIS, as of December 2019 a total of 19,730 patients with T1DM (4,021 aged <20 years and 15,709 aged ≥20 years) were registered in the NHIS for reimbursement of diabetes management expendables. Among them, 1,434 patients (7.3%) with T1DM were prescribed CGM sensors from January to December 2019. Of these individuals, 585 (40.8%) were male and 849 (59.2%) were female, while 751 (52.4%) were younger than 20 years and 683 (47.6%) were 20 years of age or older (Table 2). No sex differences were identified by age group (P=0.147). In the United States, 4% and 19% of CGM users in 2011 and 2016, respectively, were T1DM patients younger than 18 years registered in the T1DM Exchange Clinic Registry. In the Prospective Diabetes Follow-Up Registry (DPV) from Germany and Austria, 3% and 22% of the participants aged <18 years were CGM users in 2011 and 2016, respectively [33,34]. The proportion of CGM users among Korean T1DM patients younger than 20 years of age was 18.7% in 2019, which is similar to the data recorded by the T1DM Exchange Clinic Registry but slightly lower than that in the DPV registry. However, the number of actual CGM users in Korea may be underestimated because some isCGM and real-time CGM users may not have claimed reimbursement for sensors. Further, CGM users with type 2 diabetes or gestational diabetes were not included in the claim data. Nonetheless, CGM users in Korea are expected to increase continually given the introduction of partial reimbursements for CGM transmitters and insulin pumps since January 2020.

Number of patients with T1DM in Korea who were prescribed sensors for continuous glucose monitoring between January and December 2019
Successful CGM application requires education, training, and frequent contact with healthcare providers, as well as education for diabetes self-management [35,36]. Therefore, healthcare structures which provide fee-for-service educational programs delivered by experienced diabetes educators need to be improved for successful initiation and use of CGM for individuals.
Conclusion
CGM is an effective technology for facilitating better glycemic control and reducing hypoglycemia and can be used safely in the pediatric population. With recent advances in systems, CGM has become an essential consideration in management of pediatric T1DM patients. Korean CGM users are expected to increase with reimbursement of CGM sensors and transmitters by the NHIS. For successful CGM use, there is a need for implementation of a long-term policy for diabetes education and financial assistance related to CGM. Technologies for managing diabetes, including CGM, are rapidly being updated, and clinicians should acquaint themselves with interpretation of CGM data and informational updates given the importance of integrating CGM data into clinical practice among pediatric T1DM patients.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The author thanks Yoo Kyung Yoon and Sun Hye Moon of the Department of Benefits Assurance of the National Health Insurance Service, Korea, for research collaboration and access to data.