Effect of -202 A/C IGFBP-3 polymorphisms on growth responses in children with idiopathic short stature
Article information
Abstract
Purpose
This study evaluated the -202 A/C insulin-like growth factor binding protein 3 (IGFBP-3) promoter polymorphism as a predictor of serum IGFBP-3 concentration and growth velocity after recombinant growth hormone (rhGH) therapy in patients with idiopathic short stature (ISS).
Methods
Genotyping and serial measurement of clinical parameters were performed in 69 children with a confirmed diagnosis of ISS. Restriction fragment length polymorphism analysis was performed to determine the genotype at the -202 IGFBP-3 locus. Serum insulin-like growth factor 1 (IGF-1) and IGFBP-3 levels were measured at baseline and after 1 year of rhGH treatment, as were height standard deviation score and growth velocity.
Results
The -202 A/C IGFBP-3 genotype comprised 69.6% AA, 24.6% AC, and 5.8% CC. One year of treatment did not produce a meaningful difference in IGF-1 or IGFBP-3 levels between children in the AA group and those with at least one copy of the C allele (AC/CC group). Comparing the 2 groups after one year also revealed no significant difference in growth velocity (ΔHeight: 9.061±1.612 cm/yr in the AA group, 9.421±1.864 in the AC/CC group, P=0.419).
Conclusions
rhGH treatment was effective and there were no significant differences in IGF-1, IGFBP-3, or growth velocity according to genotype. Thus, -202 IGFBP-3 genotype may not be a major factor affecting individual growth responses in Korean children with ISS.
Introduction
Short stature is defined as a condition in which height is less than the corresponding mean for a given age, sex, and population group by more than 2 standard deviation scores (SDSs) [1]. There are multiple causes of short stature, such as nutritional, systemic, endocrine, or chromosomal abnormalities. Idiopathic short stature (ISS) is diagnosed when all other known causes of growth failure have been excluded, and when patients present with a normal or increased level of serum growth hormone (GH) during GH stimulation testing [1-3].
Use of recombinant human GH (rhGH) was approved in 2003 by the U.S. Food and Drug Administration to improve the final height of patients with ISS [4]. One study described increases in first-year growth velocity, height SDS, serum insulin-like growth factor I (IGF-1), and IGF-binding protein-3 (IGFBP-3) levels after rhGH treatment of children with ISS [2,5]. However, another study reported that despite the overall effectiveness of rhGH therapy, interindividual variability could occur, including a lack of response [6].
IGF-1 is an important mediator of the effects of GH. IGFBP-3 and acid labile subunits bind to IGF-1 in the bloodstream and transport IGF to target tissues. Therefore, serum IGFBP-3 level is clinically important in relation to growth. The IGFBP-3 gene is located in the 7p14-p12.11 chromosomal region. The gene's promoter region contains several single nucleotide polymorphisms (SNPs). The -202 A/C SNP located 202 bp upstream of the transcription start site consists of an A to C nucleotide change. This SNP is associated with serum IGFBP-3 concentrations in healthy adults. Serum IGFBP-3 levels are highest in patients with the AA genotype, followed by the AC and CC genotypes [7-14].
An association of the A allele in the IGFBP-3 promoter region with increased IGFBP-3 concentration and growth velocity after GH therapy has been observed in prepubertal children with GH deficiency (GHD) [15] and Turner syndrome [16]. However, IGFBP-3 polymorphisms in pediatric ISS patients have not been investigated. The present study assessed the role of -202 A/C IGFBP-3 promoter polymorphism in ISS patients treated using rhGH.
Materials and methods
1. Subjects
Children 4 to 16 years of age who underwent a GH stimulation test at Kangdong Sacred Heart Hospital from May 2014 to August 2016 were enrolled in the study. ISS diagnosis was based on a height more than 2 standard deviations (SDs) below the mean for the relevant age and sex groups; one or more incidences of peak GH levels ≥7 ng/mL on GH simulation test with 2 different stimulants (clonidine, arginine, or levodopa); no GH treatment within at least the previous 6 months; and normal thyroid hormone levels. Exclusion criteria included diagnoses of dysmorphic syndrome, skeletal dysplasia, small for gestational age (SGA), anemia, chronic disease, hypopituitarism, GHD, Cushing syndrome, or chromosomal abnormalities, or a history of use of a medication known to affect GH.
Eighty-one patients were diagnosed with ISS; of these, the-202 A/C IGFBP-3 promoter genotype was confirmed in 69 patients. Growth responses were analyzed over a 1-year period. A left-hand radiograph (including the wrist) was acquired to determine skeletal maturity, and bone age (BA) was determined by an experienced pediatric endocrinologist using the Greulich and Pyle atlas. BA was recorded in years. rhGH was administered subcutaneously at a mean dose of 0.79±0.15 IU/kg/wk for 11.9±2.7 months (median, 12.0 months). The dose was adjusted at each visit according to changes in weight.
The study protocol was approved by the Institutional Review Board (IRB) of Kangdong Sacred Heart Hospital, Seoul, Korea (IRB No. 2013-02-023). All patients or their parents provided written informed consent before participating.
2. Hormone assays
Serum IGF-1 and IGFBP-3 levels were measured by chemiluminescent immunoassay (IMMULITE 2000; Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) at the start of therapy and 1 year later. Measured values were substituted by SDS for age and sex in accordance with the serum levels for Koreans of the appropriate age and gender [17].
3. Molecular studies
Genomic DNA was extracted from blood samples from the participants. The IGFBP-3 -202 SNP was amplified from the DNA samples by polymerase chain reaction using primers 5'-CCA CGA GGT ACA CAC GAA TG-3' (forward) and 5'-AGC CGC AGT GCT CGC ATC TGG-3' (reverse), as previously described [18]. Genotyping of the IGFBP-3 locus was accomplished by restriction fragment length polymorphism analysis, as previously described [19]. Identification of the -202 A/C IGFBP-3 allele was based on expected sizes of 242 bp and 162 bp for AA; 288 bp and 162 bp for CC; and 288, 242, and 162 bp for AC.
4. Statistical analyses
Qualitative values are expressed as frequencies and percentages. Quantitative values are expressed as mean±standard deviation. Children with ISS were divided into tertile groups according to first-year growth velocity SDS (ΔHeight SDS/yr), and the frequency of the A and C alleles in each group was compared using the chi-square test. Serum IGF-1 and IGFBP-3 concentrations and the IGF-1/IGFBP-3 ratio according to -202 IGFBP-3 promoter genotype were compared using an independent sample t-test. Analysis of the therapeutic effects of GH was performed using paired t-tests. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
Results
1. Patient characteristics and genotype distribution
Sixty-nine children with ISS (37 boys and 32 girls) were evaluated. Baseline characteristics prior to treatment included chronological age (CA) of 8.5±2.9 years, BA of 6.7±3.1 years, short stature (height SDS, -2.213±0.556), body mass index (BMI) SDS of -3.149±1.398, and midparental height SDS of -0.688±0.752.
The -202 A/C IGFBP-3 SNP comprised 3 genotypes: 69.6% AA (n=48), 24.6% AC (n=17), and 5.8% CC (n=4). Since so few patients were homozygous for the C allele, we classified the patients into two -202 A/C IGFBP-3 genotype groups: the AA group and the AC/CC group. These 2 groups were statistically similar with regard to baseline chronological and BA, height SDS, BMI SDS, and mean rhGH dose (Table 1).
2. Serum IGF-1 and IGFBP-3 SDS levels
The 1-year growth response was evaluated in relation to -202 A/C IGFBP-3 genotype in 69 ISS patients. Patients in the AA allele group presented with higher IGF-1 and IGF-1 SDS levels than those in the AC/CC group before and after 1 year of treatment; however, this difference was not significant (Tables 1, 2).

First year growth response to rhGH treatment in 69 children with ISS, grouped according to the -202 A/C IGFBP-3 genotype (n=69)
IGFBP-3 concentration was significantly higher in the AA group than in the AC/CC group (4,115±1,001 vs. 3,562±879, respectively; P=0.032) before starting treatment. There was tendency toward higher IGFBP-3 SDS in the AA group, but this was not statistically significant (2,234±1,197 vs. 1,609±1,682, P=0.084) (Table 1).
In addition, levels of IGFBP-3 and IGFBP-3 SDS in the AA group after 1 year of treatment were higher than those in the AC/CC group; however, this difference was not significant (Table 2). There were no significant differences in the IGF-1/IGFBP-3 ratio between the AA group and the AC/CC group before and after 1 year of treatment (Tables 1, 2).
3. Growth velocity after one year of GH therapy
Height SDS was significantly increased after one year of GH therapy in all -202 A/C IGFBP-3 genotype groups compared to baseline height SDS (P<0.001). IGF-1 and IGFBP-3 SDS levels in both groups also increased significantly after 1 year of treatment compared to baseline levels (P<0.001). The mean height gain during the year of treatment was 9.2±1.7 cm/yr among 69 children with ISS.
BA also increased after one year of GH treatment. Mean BA was delayed beyond 1 year compared to CA before and after 1 year of treatment; however, no notable difference was observed between the AA group and the AC/CC group (Tables 1, 2). The change in BA was more advanced than the change in CA, although this difference was not significant between the 2 groups (ΔBA/ΔCA: 1.33±0.83 vs. 1.34±0.64, P=0.949) (Table 2). While first-year growth velocity was higher in the group with 1 or 2 copies of the C allele than in the homozygous A allele group, this was not significantly different (ΔHeight/year: 9.061±1.612 vs. 9.421±1.864, P=0.419) (Table 2). Similar results were observed for first-year growth velocities with SDS adjusted for sex and age (ΔHeight SDS/yr: 0.639±0.349 vs. 0.762±0.363, P=0.188) (Fig. 1A). There were also no significant differences in ΔIGF-1 SDS (1.083±1.347 vs. 0.734±0.716, P=0.269), ΔIGFBP-3 SDS (0.840±1.481 vs. 0.929±1.535, P=0.820), or ΔIGF-1/ΔIGFBP-3 (1.556±0.525 vs. 1.388±0.444, P=0.204) between the 2 groups (Fig. 1B, C).
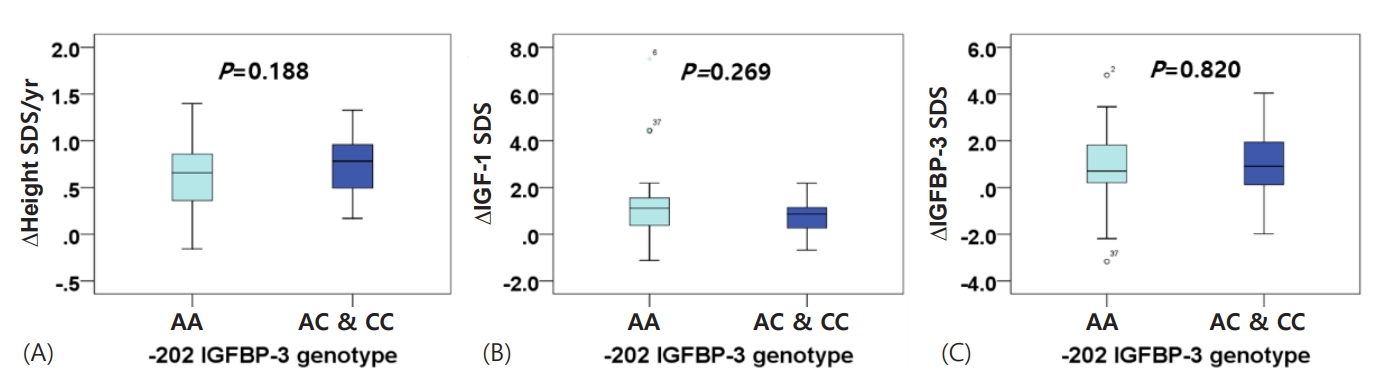
Influence of -202 A/C IGFBP-3 genotype on first-year growth velocity SDS, IGF-1 SDS levels, and IGFBP-3 SDS levels in 69 children with ISS, before and after treatment. (A) ∆Height SDS/yr, (B) ∆IGF-1 SDS, (C) ∆IGFBP-3 SDS. IGFBP-3, insulin-like growth factor binding protein 3; SDS, standard deviation score; ISS, idiopathic short stature; IGF-1, insulin-like growth factor 1.
4. Allelic frequency in relation to first-year growth velocity SDS
According to the change in height SDS from baseline to one year of rhGH treatment, all 69 patients were divided into 3 groups (n=23 per group): poor, moderate, and good. The first-year growth velocity SDS (ΔHeight SDS/yr) of patients in the poor group was less than 0.543, that of the moderate group was 0.543 to 0.812, and that of the good group was more than 0.812.
A allele frequency was 84.8% in the poor group, 82.6% in the moderate group, and 78.3% in the good group. A allele frequency decreased with increasing first-year growth velocity. The frequency of the C allele was 15.2% in the poor group, 17.4% in the moderate group, and 21.7% in the good group. C allele frequency increased as growth rate increased, but this was not statistically significant (P=0.710) (Fig. 2).

Allelic frequency of -202 A/C IGFBP-3 polymorphisms after one year of rhGH treatment in children with ISS. The 69 patients were divided into 3 groups (n=23 per group) according to first-year growth velocity SDS (∆Height SDS/ yr): poor, <0.543; 0.543 to 0.812, moderate; and >0.812, good. IGFBP-3, insulin-like growth factor binding protein 3; rhGH, recombinant growth hormone; ISS, idiopathic short stature; SDS, standard deviation score.
Discussion
In our study, the distribution of the -202 A/C IGFBP-3 genotype was 69.6% AA, 24.6% AC, and 5.8% CC (4 of 69). The A allele frequency was 82% and the C allele frequency was 18%. These results echo those from a previous study performed in Korean children [19]. Other prior studies described frequencies of the -202 A/C IGFBP-3 genotype in a European population as 16%–23% AA, 53%–64% AC, and 20%–24% CC [20,21], with frequencies of 56%–63% AA, 32%–39% AC, and 5% CC reported in East Asians [22,23], similar to this study. These results imply genotypic variation according to ethnicity or race, with the C allele being relatively prevalent in Europeans (Table 3). In a multiethnic population study, subjects were divided into quartile groups according to adult height. The mean IGFBP-3 level was higher in the A allele group than in the C allele group, and C allele frequency was higher in the taller group than in the shorter group [7]. One study reported catch-up growth after GH therapy in SGA, in which the target population was shown to have lower mean IGFBP-3 SDS level and higher mean height SDS in the C allele than in the A allele [24]. If serum IGFBP-3 levels are lower for the C allele than the A allele, serum IGF-1 binding is presumed to be low and free IGF-1 levels are presumed to be elevated. Therefore, the C allele presumably contributes to greater stature in Europeans compared to East Asians, which conforms with social norms.
GH is used to treat children with ISS (2003), GHD (1985), chronic renal insufficiency (1993), SGA (2001), and syndromic diseases such as Turner syndrome (1997), Prader-Willi syndrome (2000), and Noonan syndrome (2007) [4,5]. Children with the same cause of short stature who are treated with a similar dose of GH exhibit significant interindividual variability in short- and long-term growth responses [15,16,25,26]. Previous prediction models of the response to GH based on clinical parameters explain approximately 50% of this variability. Thus, recent interest has focused on the potential influence of genetic variation within the GH-IGF-1 axis [27-29]. IGFBP-3 is the major protein carrier for circulating IGF-1 and IGF-2, and also modulates IGF activity [30]. Deal et al. [7]. described a SNP in the IGFBP-3 promoter, which featured an A to C nucleotide change 202 bp upstream of the transcription start site (-202 A/C) near elements believed to control basal promoter activity. The authors also reported that healthy adults with an AA genotype displayed higher mean IGFBP-3 levels than levels in those with AC or CC genotypes. Comparable results have been reported in children with GHD [15] and Turner syndrome [16]. To the best of our knowledge, no prior studies have reported an association between serum IGFBP-3 levels and growth velocity according to the -202 A/C IGFBP-3 polymorphism in children with ISS.
In the present study, there were no significant differences in first-year growth responses to GH therapy between the AA group and the AC/CC group. Furthermore, there was no correlation between genotypes and serum IGFBP-3 levels. Serum IGFBP-3 level was slightly higher and first-year growth velocity was lower in the AA group than in the AC/CC group. There appears to be a difference in C allele frequency depending on ethnicity and race, and estimated serum-free IGF-1 levels were relatively elevated for the C allele group, which may explain the greater growth. Serum-free IGF-1 level was estimated based on the IGF-1/IGFBP-3 ratio. However, IGF-1/IGFBP-3 ratio was similar among genotypes. In a previous study, serum IGFBP-3 levels differed significantly depending on genotype; however, the IGF-1/IGFBP-3 ratio was not different [14,19]. These findings suggest that serum IGF-1 concentrations reflect neither systemically nor locally produced IGF-1, and that variation at the -202 IGFBP-3 locus does not directly affect serum IGF-1 or free IGF-1 levels.
GH therapy resulted in a significant increase in first-year growth velocity and mean first-year growth velocity SDS compared to baseline. These results are consistent with prior studies. In one study, first-year growth velocity (10.68±1.95 cm/yr) and ΔHeight SDS (0.63±0.16) increased significantly after 6 months of GH therapy in Korean children with ISS [5]. Other studies reported an increased velocity of 8–9 cm/yr in the first year of GH treatment compared with 4–5 cm/yr before treatment [31,32]. The BMI SDS of children with ISS was less than 2 SD in our study. Wudy et al. [33]. reported that decreased appetite and lower BMI in ISS patients contribute to their short stature. In addition, BMI reportedly modulates the IGF-1 response to GH administration in children with ISS, suggesting that GH sensitivity may be affected by nutritional status [34].
Our study has some limitations. First, -202 A/C IGFBP-3 genotypes could not be divided into 3 groups because of the low frequency of the CC genotype in the Korean population. Second, the sample size was relatively small. The CC genotype represented 25% (18 of 71) of the population in the GHD [15] study and 35% (39 of 112) in the Turner syndrome study [16]. IGFBP-3 levels and growth velocities between these 2 studies statistically differed depending on genotype. Therefore, increasing the sample size or selecting an ethnicity with a high frequency of the CC genotype as the target group may help in obtaining effective results. Third, serum IGF-1, and IGFBP-3 levels do not accurately reflect concentrations in local tissues, especially growth plates. Despite these limitations, this study is noteworthy as it is the first attempt to evaluate the impact of -202 A/C IGFBP-3 polymorphisms in children with ISS. Further studies that include a larger number of ISS patients are warranted.
In conclusion, we evaluated the possible association of IGF-1, IGFBP-3, and growth velocity with -202 A/C IGFBP-3 promoter polymorphisms in children with ISS. GH treatment was effective and there were no significant differences in IGF-1, IGFBP-3, or growth velocity according to genotype. Thus, -202 IGFBP-3 genotype may not be a major factor affecting interindividual growth response in Korean children with ISS. Since the frequency of the C allele varies by ethnicity and race, further studies in different populations should be conducted to definitively determine the role of this polymorphism.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was financially supported by a research grant from the Investigator-Initiated Trials program of Dong-A ST Co., LTD. Opinions expressed in this paper are those of the authors and do not represent those of Dong-A ST Co., LTD. The authors would also like to thank Dong-A ST Co., LTD for supporting this research.


