 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 24(2); 2019 > Article |
|
Abstract
Recombinant human growth hormone (GH) has been in use for over 30 years, and its indications have gradually expanded from the classical replacement therapy in GH deficiency (GHD) to pharmacological therapy in patients with normal GH secretion. The insulin-like growth factor-I (IGF-I ) is closely GH dependent and is the effector of GH biological actions in peripheral tissues. Since IGF-I has potent mitogenic and antiapoptotic effects, the use of GH, especially outside GHD, has raised safety concern regarding cancer risk. The results of experimental, epidemiological and observational studies are not univocal and a number of biases and confounders affect the interpretation of data. The aim of this review is to critically review the data linking GH therapy during childhood with cancer risk, highlighting strengths and weaknesses of the available evidence.
Recombinant human growth hormone (r-hGH) was introduced in the market in 1985 for the treatment of children with growth hormone deficiency (GHD). Thereafter, due to the virtually unlimited supply of r-hGH, the indications for its use have progressively expanded worldwide to include patients with normal growth hormone (GH) secretion (Table 1). Therefore, r-hGH therapy has changed over the years from a classical replacement therapy to a pharmacological treatment. The use of r-hGH in patients with normal GH secretion has raised concerns about the safety of such therapy. The final mediator of the growth promoting action of GH is insulin-like growth factor-I (IGF-I), which exerts potent antiapoptotic and mitogenic activity in all cells of the organism and is expressed and secreted from many different types of cancer cells. However, while there is strong evidence for a role of the GH-IGF axis in the development, maintenance, and spread of tumors based on experimental data obtained in cellular and animal models, such evidence is weak in humans [1].
The aim of this review is to provide an up-to-date critical review of data linking GH therapy during childhood with cancer risk, highlighting strengths and weaknesses of the available evidence.
GH is the main regulator of hepatic IGF-I production. IGF-I, in turn, modulates GH release in a negative feedback loop and mediates the growth promoting action of GH in cartilage [2]. IGF-I and IGF-II comprise a family of peptides structurally related to insulin that promote cell growth through interaction with specific high affinity receptors [3]. Circulating IGFs are synthesized primarily in the liver and exert an endocrine function. There is good correlation between growth and IGF-I levels [4]. and data from IGF-I transgenic mice have definitively proved the growth-promoting action of IGF-I in vivo [5]. IGF-I is also produced by most, if not all, tissues and acts in an autocrine-paracrine mode [4]. Both serum and tissue IGFs are bound to specific binding proteins (IGFBPs) and six structurally different IGFBPs (termed IGFBPs 1–6) have been identified and sequenced [6,7]. IGFBPs function not only as carriers but also modulate the release of IGFs to the tissues [7,8]. IGFs circulate in three different forms: unbound (free), in binary complexes with IGF-binding protein (IGFBP) 1 to 6, and in ternary complexes containing an IGFBP and the approximately 85-kDa glycoprotein known as the acid-labile subunit (ALS) [9]. The circulating half-lives of IGF-I and IGF-II are reported to be about 10 minutes in the free form, less than 30 minutes in binary complexes, and 12–15 hours in the ternary complexed form [10]. IGFBP-3 has been extensively documented as being unique among the IGFBPs in its ability to form ternary complexes with the IGFs and ALS [11]. These complexes form a circulating reservoir of IGFBP-3 and IGFs as, unlike free and binary complexed IGFs, they do not cross the capillary barrier [12]. IGFBP-5, like IGFBP-3, is able to form heterotrimers by combining with IGF-I or IGF-II and ALS [13].
IGFBPs, in turn, are under control of specific proteases which, by fragmenting the IGFBP molecule, regulate the affinity and, consequently, the release of IGFs from IGFBPs [14]. In peripheral tissues, IGFs bind to specific cell surface receptors, which mediate their mitogenic and anti-apoptotic effects [3,11]. The IGF-I receptor is the main mediator of the biological actions of both IGF-I and IGF-II. The insulin receptor mediates some of the IGF-II cellular effects [15]. The IGF-II/M-6-P receptor does not play any role in IGF signal transduction but is responsible for clearing, and thereby reducing, the circulating levels of IGF-II [16]. Fig. 1 shows the IGF system.
The GH/IGF-I axis activation seems to play a role in different types of cancer. Prostate, breast, endometrial and colorectal neoplasms express both GH and GH receptor and other tumors express GH releasing hormone [1]. Furthermore, the overexpression of GH increases proliferation and promotes survival of different cancer cell lines [17-19].
The IGFs are recognized as important growth factors in many tumor cell types [20] and virtually all human cancer cells express IGF receptors [21] and produce IGFs [22], IGFBPs [23], and IGFBP proteases [24]. In other words, many tumors locally create a specific IGF system that sustains their own growth and dissemination. The type I IGF receptor is actively involved in the control of cell growth and differentiation, and mutations of this receptor inhibit the development of tumors in mice [25]. Finally, there is increasing evidence that IGFs are also able to stimulate cell motility. Many types of cells, such as endothelial cells, keratinocytes, osteoblasts, rhabdomyosarcoma cells, epithelial cells, trophoblasts, melanoma cells, breast cancer cells, smooth muscle cells, carcinoma cells and neuroblastoma cells, migrate toward a source of IGFs or display increased motility in the presence of these factors [26,27]. Taken together, these findings seem to confirm the original hypothesis of Sporn and Roberts [28] who first suggested that growth factors play a pivotal role in cancer development and growth.
Whilst the strength of evidence for a relationship between GH/IGF-I axis and cancer risk is high in both cellular and animal models, data in humans are scarce and conflicting.
Epidemiological studies have shown an association between elevated circulating levels of IGF-I and an increased risk of developing certain cancers such as prostate, breast and colorectal neoplasms. (Box 1) [33-37] The association between GH-IGF and carcinogenesis is also suggested by the observation that patients suffering from acromegaly, an endocrine disorder characterized by sustained hypersecretion of GH and consequent increased levels of IGF-I, have a higher risk of developing colorectal and thyroid cancer [38-42]. On the other side, patients with either primary or secondary IGF-I deficiency seem to be protected from developing malignancies [43-45].
Overall these data indicate an association, not a causal relationship. Genetic predisposition as well as common nutritional and environmental factors may in fact account for these associations.
The first report suggesting a potential link between GH treatment and malignancy dates back to the '80s when leukemia risk was associated with the use of GH [46]. Further detailed analysis of these cases revealed that these patients had concomitant conditions predisposing them to cancer independently of GH therapy. The risk of leukemia was not increased in children treated with GH in the United States according to the National Cooperative Growth study initiated in 1985 [47]. In 2002, a long-term study of 1,848 patients treated with human pituitary GH during childhood and early adulthood showed an increased risk of colorectal cancer and Hodgkin lymphoma (HL) [48]. However, it has to be pointed out that the absolute number of recorded deaths for colorectal cancer was 2 against an expected number of 0.18 and the absolute number of cases with colorectal cancer and HL was 2 against an expected number of 0.13. Therefore, in spite of the statistical significance, the absolute number of cases was extremely low. Nevertheless, although based on small numbers, the observed risk of neoplasms raised some concern and paved the way for further long-term observational studies.
In 2014 we performed a systematic review and meta-analysis of published studies reporting data on long-term impact of r-hGH therapy on the risk of cancer mortality and morbidity in patients treated during childhood. Three studies reporting the standard mortality ratio (SMR) [49-51], three reporting the standard incidence ratio (SIR) [47,52,53] and 3 reporting the overall relative risk (RR) of second neoplasms [54-56], were analyzed. The results showed that malignancy SMR was not significantly increased whereas overall cancer SIR (2.74; 95% confidence interval [CI], 1.18–4.41) and the RR for second neoplasms (1.99; 95% CI, 1.28–3.08) were significantly increased [57]. However, all the studies analyzed were affected by a number of confounders and biases such as: (1) the heterogeneity of the study populations comprising both adult and pediatric cohorts and including patients with different diagnoses; (2) the limited sample size; (3) the low event rate; (4) the lack of an untreated control group; (5) the lack of key data such as familial predisposition to diseases and exposure to environmental hazards; (6) the lack of local mortality and morbidity indices; (7) the lack of information on dose and treatment duration.
In 2012, two independent studies on long-term mortality in patients treated with r-hGH during childhood, published in the same issue of the same journal [51,58], reported opposite results. The French study showed a significant increase in mortality for bone tumors in a cohort of about 6,500 young adult subjects treated with r-hGH during childhood for the indications of isolated GH deficiency (IGHD), short stature associated with small for gestational age (SGA), or idiopathic short stature (ISS) [51]. The other study involving cohorts from Sweden, The Netherlands, and Belgium, reported not a single case of death from cancer in about 2,500 subjects with the same diagnostic categories [58].
A more recent Swedish study applied a novel mortality model using continuous hazard-functions adjusting for birth-characteristics, gender, age-intervals, and calendar-year to estimate SMR in a population of 3,847 patients diagnosed with IGHD (n=1,890), ISS (n=975), and SGA (n=982). Compared with the general Swedish population, the ratio of observed/expected deaths (21/21.99) was not increased in childhood r-hGH-treated IGHD, ISS, and SGA-patients after adjusting for birth-characteristics [59].
In the GeNeSIS (Genetics and Neuroendocrinology of Short Stature International Study) prospective, multinational, observational study sponsored by Eli Lilly and conducted on 9,505 GH-treated patients with different diagnoses followed for at least 4 years, no significant increase in cancer mortality was observed in IGHD, ISS, and SGA patients [60]. These findings have recently been confirmed in a further analysis conducted on over 20,000 GeNeSIS patients [61].
A position paper delivered by the international scientific societies involved in the use of r-hGH in both children and adults, stated that the available evidence in children does not indicate either an increased risk of new primary cancers or an increased risk of recurrence of primary cancer in GH recipients [62]. The risk of second neoplasms in GH-treated survivors of pediatric cancers seems to be increased but declining with longer follow-up [55,62-64].
Most observational studies conducted in patients treated with r-hGH have been based on pharmaceutical databases and have reported short-term follow-up results. The SAGhE (Safety and Appropriateness of Growth Hormone Treatments in Europe) study was conceived to provide a large-scale European collaborative cohort study of r-hGH-treated patients with long-term follow-up for cancer incidence and mortality, independently of pharmaceutical companies.
The SAGhE study recruited cohorts of patients from 8 European countries treated in childhood with r-hGH. The study population consisted of 24,232 patients and represents the largest and longest follow-up cohort of GH-treated patients with follow-up and analysis, independent of industry [65].
The SAGhE population was classified according to the initial diagnoses into three different classes of cancer risk: (1) low risk: isolated growth failure, including IGHD, ISS and SGA; (2) high risk: cancer, including patients with previous history of cancer; (3) intermediate risk: nonisolated growth failure and non-cancer patients, including all the other patients such as multiple pituitary deficiencies, Turner syndrome, Noonan syndrome and bone dysplasias. The cohort for cancer related mortality risk comprised 23,984 patients and for cancer incidence 10,406 patients. The average length of follow-up for mortality was 16.5 years per patient, and for cancer incidence 14.8 years per patient. The mean age at the end of follow-up was 27.1 years for the cancer mortality analysis and 25.8 years for the incidence analysis [66].
In the low risk (isolated growth failure) group, both mortality and morbidity for cancer was not increased. SMR and SIR for many types of cancers were significantly increased in GH-treated patients of the high-risk group. In the intermediate risk group, the incidence of bone and bladder cancers was significantly raised in GH-treated patients. Cancer risk was unrelated to the duration or cumulative dose of r-hGH treatment, but for patients treated after previous cancer, cancer mortality risk increased significantly with increasing daily r-hGH dose. Finally, the HL incidence increased significantly with longer follow-up (P trend=0.001 for patients overall and 0.002 for patients without previous cancer) [66].
Although the SAGhE study is more accurate and informative than most previous observational studies, it nonetheless shares a number of biases and confounders that affect the interpretation of results (Box 2) and, although more informative than most previous observational studies, even the SAGhE has intrinsic weaknesses that limit its epidemiological and clinical value (Box 3).
The available evidence does not indicate an increased risk of cancer within the length of follow-up currently available especially for low risk children (IGHD, ISS, and SGA). However, it has to be pointed out that data from the French cohort of the SAGhE population indicate an increased risk for bone tumor mortality and morbidity even in the low risk cohorts [51,67]. The available data suggest that r-hGH treatment does not substantially increase leukemia risk in patients without prior high risk, but it is unclear whether the risk is increased in high-risk individuals [46,47,66]. Increased risk of mortality for HL in children treated with pituitary derived GH was reported [48]. This finding was not confirmed in the SAGhE study though a significant trend with longer follow-up (although no trend with GH dose) was observed [66].
Although overall the results from the observational studies are reassuring, a number of biases, confounders and weaknesses limit the value and interpretation of all data reported so far. The use of r-hGH as replacement treatment to accelerate growth and improve adult height appears fully justified in conditions where therapy replaces an unequivocal deficiency. The pharmacological use of r-hGH in short children with sufficient GH secretion raises the issue of benefit against potential adverse effects and cost to the healthcare system. The establishment and follow-up of lifespan cohorts consisting of patients treated with GH during childhood, adolescence, and adult life would be needed to properly address the issue of long-term GH safety. Such cohorts should be characterized by: (1) adequate sample size and statistical power; (2) careful characterization of patients relative to the underlying disorder (including etiology, severity, genetic syndromes, comorbidities, and response to treatment), as well as socio-demographics; (3) accurate documentation of GH treatment and response; (4) measurement of IGF-I concentrations; (5) comprehensive long-term surveillance, including documentation of all adverse outcomes; and (6) last, but not least, an appropriate control group [68,69].
Fig. 1.
The IGF system. IGF, insulin-like growth factor; IGF1R, IGF 1 receptor; IGF2R, IGF 2 receptor; M6PCI, mannose-6-phosphate cation independent; IGFBP, insulin-like growth factor binding protein; ALS, acid-labile subunit.
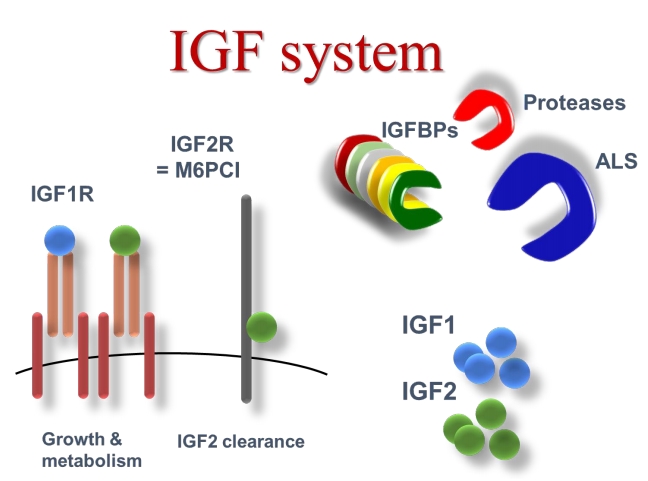
Table 1.
Approved indications for recombinant human growth hormone
References
1. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol 2011;7:11–24.



3. LeRoith D, Werner H, Beitner-Johnson D, Roberts CT Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev 1995;16:143–63.



4. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev 2001;22:53–74.



5. Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993;75:73–82.


6. Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog Growth Factor Res 1991;3:243–66.


7. Cianfarani S, Holly JM. Somatomedin-binding proteins: what role do they play in the growth process? Eur J Pediatr 1989;149:76–9.



8. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 1999;20:761–87.


9. Baxter RC. Circulating binding proteins for the insulinlike growth factors. Trends Endocrinol Metab 1993;4:91–6.


10. Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989;121:753–8.


11. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995;16:3–34.


12. Binoux M, Hossenlopp P. Insulin-like growth factor (IGF) and IGF-binding proteins: comparison of human serum and lymph. J Clin Endocrinol Metab 1988;67:509–14.



13. Twigg SM, Baxter RC. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem 1998;273:6074–9.


14. Cianfarani S, Germani D, Rossi P, Spagnoli A, Mercanti D. Do insulin-like growth factor binding proteins (IGFBPs) modulate the IGF-I growth promoting and differentiating effects in human neuroblastoma cells? Eur J Endocrinol 1996;135:716–23.


15. Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol 1997;189:33–48.


16. Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 1992;61:307–30.


17. Kaulsay KK, Mertani HC, Tornell J, Morel G, Lee KO, Lobie PE. Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp Cell Res 1999;250:35–50.


18. Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE. Phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc Natl Acad Sci U S A 2004;101:15166–71.



19. Zhu T, Starling-Emerald B, Zhang X, Lee KO, Gluckman PD, Mertani HC, et al. Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res 2005;65:317–24.



20. LeRoith D, Baserga R, Helman L, Roberts CT Jr. Insulin-like growth factors and cancer. Ann Intern Med 1995;122:54–9.



21. Daughaday WH. The possible autocrine/paracrine and endocrine roles of insulin-like growth factors of human tumors. Endocrinology 1990;127:1–4.



23. Reeve JG, Kirby LB, Brinkman A, Hughes SA, Schwander J, Bleehen NM. Insulin-like growth-factor-binding protein gene expression and protein production by human tumour cell lines. Int J Cancer 1992;51:818–21.


24. Frost VJ, Macaulay VM, Wass JA, Holly JM. Proteolytic modification of insulin-like growth factor-binding proteins: comparison of conditioned media from human cell lines, circulating proteases and characterized enzymes. J Endocrinol 1993;138:545–54.


25. Blakesley VA, Kalebic T, Helman LJ, Stannard B, Faria TN, Roberts CT Jr, et al. Tumorigenic and mitogenic capacities are reduced in transfected fibroblasts expressing mutant insulin-like growth factor (IGF)-I receptors. The role of tyrosine residues 1250, 1251, and 1316 in the carboxy-terminus of the IGF-I receptor. Endocrinology 1996;137:410–7.



26. Leventhal PS, Feldman EL. Insulin-like growth factors as regulators of cell motility signaling mechanisms. Trends Endocrinol Metab 1997;8:1–6.


27. Puglianiello A, Germani D, Rossi P, Cianfarani S. IGF-I stimulates chemotaxis of human neuroblasts. Involvement of type 1 IGF receptor, IGF binding proteins, phosphatidylinositol-3 kinase pathway and plasmin system. J Endocrinol 2000;165:123–31.


29. Swanson SM, Unterman TG. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis 2002;23:977–82.



30. Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology 2002;143:4139–42.


31. Yang XF, Beamer WG, Huynh H, Pollak M. Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res 1996;56:1509–11.

32. Tornell J, Rymo L, Isaksson OG. Induction of mammary adenocarcinomas in metallothionein promoter-human growth hormone transgenic mice. Int J Cancer 1991;49:114–7.


33. Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 1998;279:563–6.


34. Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 2000;9:345–9.

35. Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998;351:1393–6.


36. Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 1999;91:620–5.



37. Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res 2016;76:2288–300.



38. Baris D, Gridley G, Ron E, Weiderpass E, Mellemkjaer L, Ekbom A, et al. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control 2002;13:395–400.


39. Kauppinen-Makelin R, Sane T, Valimaki MJ, Markkanen H, Niskanen L, Ebeling T, et al. Increased cancer incidence in acromegaly--a nationwide survey. Clin Endocrinol (Oxf) 2010;72:278–9.


40. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 1998;83:2730–4.

41. Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF Jr. Acromegaly and gastrointestinal cancer. Cancer 1991;68:1673–7.


43. Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res 2007;17:54–7.


44. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against postnatal development of malignancies. Eur J Endocrinol 2011;164:485–9.


46. Watanabe S, Mizuno S, Oshima LH, Tsunematsu Y, Fujimoto J, Komiyama A. Leukemia and other malignancies among GH users. J Pediatr Endocrinol 1993;6:99–108.



47. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab 2010;95:167–77.



48. Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959-85: a cohort study. Lancet 2002;360:273–7.


49. van Bunderen CC, van Nieuwpoort IC, Arwert LI, Heymans MW, Franken AA, Koppeschaar HP, et al. Does growth hormone replacement therapy reduce mortality in adults with growth hormone deficiency? Data from the Dutch National Registry of Growth Hormone Treatment in adults. J Clin Endocrinol Metab 2011;96:3151–9.


50. Gaillard RC, Mattsson AF, Akerblad AC, Bengtsson BA, Cara J, Feldt-Rasmussen U, et al. Overall and cause-specific mortality in GH-deficient adults on GH replacement. Eur J Endocrinol 2012;166:1069–77.


51. Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab 2012;97:416–25.



52. Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr 2010;157:265–70.


53. Child CJ, Zimmermann AG, Woodmansee WW, Green DM, Li JJ, Jung H, et al. Assessment of primary cancers in GH-treated adult hypopituitary patients: an analysis from the Hypopituitary Control and Complications Study. Eur J Endocrinol 2011;165:217–23.



54. Woodmansee WW, Zimmermann AG, Child CJ, Rong Q, Erfurth EM, Beck-Peccoz P, et al. Incidence of second neoplasm in childhood cancer survivors treated with GH: an analysis of GeNeSIS and HypoCCS. Eur J Endocrinol 2013;168:565–73.


55. Ergun-Longmire B, Mertens AC, Mitby P, Qin J, Heller G, Shi W, et al. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab 2006;91:3494–8.



56. Mackenzie S, Craven T, Gattamaneni HR, Swindell R, Shalet SM, Brabant G. Long-term safety of growth hormone replacement after CNS irradiation. J Clin Endocrinol Metab 2011;96:2756–61.


57. Deodati A, Ferroli BB, Cianfarani S. Association between growth hormone therapy and mortality, cancer and cardiovascular risk: systematic review and meta-analysis. Growth Horm IGF Res 2014;24:105–11.


58. Savendahl L, Maes M, Albertsson-Wikland K, Borgstrom B, Carel JC, Henrard S, et al. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab 2012;97:E213–7.


59. Albertsson-Wikland K, Martensson A, Savendahl L, Niklasson A, Bang P, Dahlgren J, et al. Mortality Is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab 2016;101:2149–59.


60. Quigley CA, Child CJ, Zimmermann AG, Rosenfeld RG, Robison LL, Blum WF. Mortality in children receiving growth hormone treatment of growth disorders: data from the genetics and neuroendocrinology of short stature international study. J Clin Endocrinol Metab 2017;102:3195–205.



61. Child CJ, Zimmermann AG, Chrousos GP, Cummings E, Deal CL, Hasegawa T, et al. Safety outcomes during pediatric GH therapy: final results from the prospective GeNeSIS observational program. J Clin Endocrinol Metab 2019;104:379–89.



62. Allen DB, Backeljauw P, Bidlingmaier M, Biller BM, Boguszewski M, Burman P, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol 2016;174:P1–9.


63. Sklar CA, Mertens AC, Mitby P, Occhiogrosso G, Qin J, Heller G, et al. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 2002;87:3136–41.


64. Patterson BC, Chen Y, Sklar CA, Neglia J, Yasui Y, Mertens A, et al. Growth hormone exposure as a risk factor for the development of subsequent neoplasms of the central nervous system: a report from the childhood cancer survivor study. J Clin Endocrinol Metab 2014;99:2030–7.




65. Swerdlow AJ, Cooke R, Albertsson-Wikland K, Borgstrom B, Butler G, Cianfarani S, et al. Description of the SAGhE Cohort: a large European Study of mortality and cancer incidence risks after childhood treatment with recombinant growth hormone. Horm Res Paediatr 2015;84:172–83.



66. Swerdlow AJ, Cooke R, Beckers D, Borgstrom B, Butler G, Carel JC, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European Cohort Study. J Clin Endocrinol Metab 2017;102:1661–72.




67. Poidvin A, Carel JC, Ecosse E, Levy D, Michon J, Coste J. Increased risk of bone tumors after growth hormone treatment in childhood: a population-based cohort study in France. Cancer Med 2018;Jun 14 [Epub]. https://doi.org/10.1002/cam4.1602.

Box 1. Key background points
• GH treatment causes exposure of tissues to increased GH and IGF-I levels.
• GH and IGF-I have the potential to promote tumor growth and progression (in cellular and animal models) but do not lead to abnormal differentiation of cells.
• Acromegaly, a condition characterized by long-standing excess GH secretion, is associated with increased risk of colorectal and thyroid cancers.
• Cumulative evidence from epidemiological studies supports an association between elevated circulating levels of IGF-I and an increased risk of certain cancers.
Box 2. Biases and confounders in all available studies
• No comparison data for untreated patients.
• Insufficient duration and completeness of follow-up.
• Multicenter study (missing data, unidentified confounders).
• Population heterogeneity (age, diagnosis).
• Limited sample size.
• Low event rate.
• Lack of key data such as familial predisposition to certain diseases, exposure to environmental hazards, lack of local mortality and morbidity indices.
Box 3. Biases and confounders in all available studies
• No information on GH treatment beyond pediatric ages, so treatment duration for some patients may have been underestimated with consequent dilution of any true effect of duration on cancer risk.
• Aggregation of data from eight (SAGhE) countries adds to the complexity of heterogeneity in patient mix and treatments.
• No information on IGF-I levels.
• Follow-up included few person-years beyond age 35, and hence had limited power for cancers prevalent at and past middle age.
• It is important to distinguish SMR from other metrics such as absolute risk and number-needed-to- harm, with the latter being more relevant to counselling patients and families about risk.
- TOOLS
- Related articles in APEM
-
Growth hormone treatment in non-growth hormone-deficient children2014 March;19(1)
Gitelman syndrome combined with complete growth hormone deficiency2013 March;18(1)
Genetic syndromes associated with overgrowth in childhood2013 September;18(3)
Recurrence of Brain Tumors in Patients Treated with Growth Hormone.2007 December;12(2)







