|
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 24(1); 2019 > Article |
|
Abstract
Hypothalamic hamartoma (HH) is one of the most common causes of central precocious puberty (CPP) in first few years of life. It can present with either seizures or CPP, although both manifestations coexist in the majority of the children. Gelastic seizures (GS), or laughing spells, are usually the first type of seizures seen in patients with HH. Although a wide variety of seizure types are known to occur in children with HH, GS are most common and consistent seizure type. The clinical presentation of HH may vary with the size and position of the mass, although large tumours typically present with both CPP and seizures. Although CPP can be managed with medical therapy, seizures can be very difficult to treat, even with multiple antiepileptic drugs. Noninvasive gamma knife surgery has been used with some success for the treatment of refractory epilepsy. We present a case of HH with very early onset seizures and CPP. The patient had an atypical form of seizures described by the parents as a "trance-like state" in which the patient had prolonged episodes of unresponsiveness lasting for hours with normal feedings during the episodes. GS occurred late in the course and were refractory to various combinations of antiepileptic drugs. A brain magnetic resonance imaging showed a large sessile HH (>20 mm). Later in the course of the disease, the patient experienced cognitive and behavioural problems. The patient underwent gamma knife surgery at nearly 5 years of age and experienced a modest response in seizure frequency. This case highlights the presentation of HH as a previously unreported seizure morphology described as a prolonged "trance-like state."
Gelastic seizures (GS) are described as a sensation of pleasant feelings or a prolonged pressure to laugh or smile. GS are considered a pathognomonic manifestation of hypothalamic hamartoma (HH), and GS are usually the first type of seizures in children with HH [1]. The age of onset of GS in HH ranges from 1 month to 15 years, and the mean age of presentation is 2.8 years [2]. Although other types of seizures have been reported in HH, they usually occur later in the course of the disease. Central precocious puberty (CPP), commonly associated with HH, starts at an average age of 3.7 years in boys and 2.5 years in girls [3]. Here, we present a boy with an atypical presentation of HH: episodes of prolonged unresponsiveness starting in the first month of life and early onset CPP.
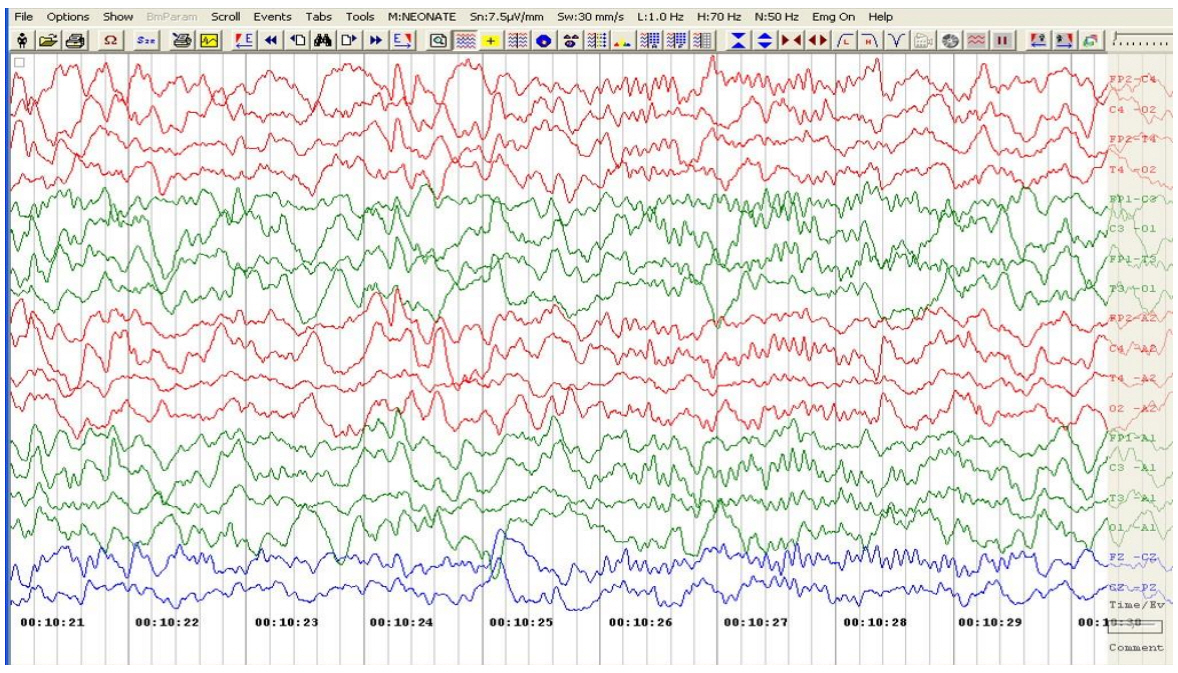
A 5-month-old male, born at full-term gestation weighing 3,300 g without any antenatal or perinatal problems, presented to our hospital with episodes of unresponsiveness that lasted 2 to 12 hours since day 4 of life. The episodes occurred almost daily and were characterized by unresponsiveness to painful stimuli, no social interaction, and no eye contact, although the patient did accept breast feeds during the episode. A brief abnormal shrill cry preceded the episodes, which the parents described as a "trance-like state" with no response to any external stimuli. In addition, the patient appeared to be awake with wide-open eyes, but he seemed to be disconnected from the outer world. He did not have any hunger-cry during these episodes, even when these episodes lasted up to 12 hours. The patient experienced a total of 3 episodes of brief multifocal seizures over next 18 days (day 7 to day 24 of life), when the baby was admitted to a local hospital for evaluation. There was no history of birth asphyxia or fever, and the patient remained well during the seizure-free intervals. Laboratory investigations, including blood sugar, serum calcium, sepsis screen, serum vitamin D, and parathyroid hormone levels, were normal. An ultrasound of the brain was normal. The patient was given phenobarbitone, phenytoin, and pyridoxine. An electroencephalogram (EEG) performed at 4.5 months of age did not reveal any abnormalities, so phenytoin was stopped, although the patient continued to take phenobarbitone. The patient was exclusively breast fed, and he had normal developmental milestones until 5 months of age. There was no family history of epilepsy. Clinical examination at 5 months revealed normal weight (50th–75th percentile), length (25th–50th percentile), and head circumference (40 cm; 0 to –2SDS [Standard deviation Score]) (Table 1). During a "trance-like state" episode in the hospital, the patient appeared as described above without tachycardia, tachypnoea, or low oxygen saturation on pulse oximeter. Genital examination at five months of age showed pubic hair at Tanner stage II, genitalia at Tanner stage III, bilateral testicular volume of 5 mL and stretched penile length of 4.5 cm. Neurological and other system examinations were normal. Bone age was significantly advanced to 18–24 months (at chronological age of 5 months). Serum testosterone (baseline, 9 am) was 0.373 nmol/L (normal prepubertal levels; <0.1–0.35). A gonadotropin-releasing hormone (GnRH) analogue (leuprolide) stimulation test resulted in the serum luteinizing hormone (LH) increasing from 0.9 to 19.3 and follicle stimulating hormone increasing from 0.559 to 1.67 IU/L (electrochemiluminescence immunoassay), suggesting an active hypothalamic-pituitary gonadotropin axis. A repeat EEG done at 5 months of age also did not show any remarkable findings (Fig. 1).
Because of the patient's enlarged testes, seizures, and positive GnRH analogue stimulation test, a diagnosis of gonadotropin dependent precocious puberty was considered. A magnetic resonance image (MRI) of the brain showed a large, relatively homogenous, nonenhancing, lobular, mass lesion in the suprasellar cistern, suggesting HH (Fig. 2). The location and size of the lesion was consistent with type III HH, as described by Delalande and Fohlen [4]. The patient was given a long-acting GnRH analogue (injection leuprolide depot 3.75 mg intramuscular, 3 monthly i.e., 0.2 mg/kg/month). The oral phenobarbitone was continued at 5 mg/kg/day.
Follow-up revealed that the episodes of unresponsiveness stopped for few months and then started again with decreased frequency and duration. In addition, typical GS started at 13 months of age. Because the pubertal progression continued, the dosage of leuprolide was increased to 11.25 mg every 10 weeks until 18 months of age after which his serum LH was optimally suppressed (Table 1). The patient experienced developmental delay with bisyllable speech appearing at 15 months and independent walking at 20 months. By 18 months of age, his pubertal progression stopped, but he continued to have episodes of unresponsiveness and GS. The patient's seizure frequency increased, so the phenobarbitone was stopped and his anti-epileptic medications were sequentially changed to levetiracetam and oxcarbazepine. Clobazam was also added after a few months (Table 1). This treatment regimen continued for 3 years; the patient’s physical growth was normal and his daily physical activities remained unaffected, although the GS continued. Follow-up details are summarized in Table 1.
At 5 years of age, the patient's seizure frequency increased and he performed poorly in school. Because the patient was already on multiple antiepileptics, treatment with gamma knife surgery was discussed with the parents. The patient underwent a repeat MRI of the brain, and gamma knife surgery was performed. The size of the HH was large, so gamma knife surgery was performed in 3 settings over 3 consecutive days. The seizures worsened for a week postoperatively but returned to the preoperative state over a 2-week period. Three months after the gamma knife surgery, there has only been marginal improvement in seizure frequency. The patient continues on antiepileptics and the GnRH analogue with a plan to follow-up for clinical response and repeat the MRI brain to evaluate a possible reduction in tumour size.
HH is defined as a non-neoplastic developmental (5th–6th week of gestation) malformation that appears in and/or around the hypothalamus between the infundibular recess and the mammillary bodies. Pathologically, HH consists of mature neurons intermingled with glial cells, and the usual size is 10–30 mm [5]. Its prevalence is estimated to be 1–2 cases per 1,000,000 children and adolescents [6].
The present case includes some interesting aspects. The patient experienced episodes of prolonged unresponsiveness lasting up to 12 hours starting in the first few weeks after birth and also early onset precocious puberty. The episodes were described as a "trance-like-state" or "loss of contact" with the environment without any crying or cooing response to even painful stimuli, although the patient did feed well during these episodes. Another unusual feature was the early onset of 'nongelastic' seizures starting at day 7 of life. The seizures were multifocal clonic movements consistent with the semiology of metabolic seizures. Although the progression of CPP could be halted after 9–12 months of therapy, the seizures were very difficult to control despite the use of multiple antiepileptics. Eventually, the patient had to undergo gamma knife surgery.
The clinical symptoms of HH depend on the size and location of the lesion in the hypothalamic-pituitary region. The "trance-like state" episodes could be early manifestations of behavioural and cognitive problems observed in relatively older children with large sessile HH, as in our case [7,8]. Cognitive impairment (language delay and learning disability) and behavioural disorders (attention deficit hyperactivity disorder or ADHD, aggressiveness, anxiety, oppositional defiant disorder, etc.) are a constant feature observed in patients with HH and epilepsy. A retrospective analysis of 60 patients reported 81.6% of patients had mental impairment, and 34% of patients suffered behavioural disturbances [2]. Cognitive or developmental impairment was reported in 43% of patients in another study of 100 cases of HH [8]. Our patient had features of cognitive impairment in the form of speech delay along with poor school performance and stubborn behaviour.
The pedunculated lesions of HH are more likely to be associated with predominantly endocrine symptoms (precocious puberty and obesity). Similarly, anteriorly placed lesions in contact with the pituitary stalk and parahypothalamic lesions are more likely to result in precocious puberty. As in our case, large HH lesions (>20 mm) are likely to present with both non-GS with associated cognitive impairment and CPP [8].
The GS started relatively late in the course of the present patient's disease, and he experienced non-GS very early as the first type of seizures. However, the sequence of seizure type is usually converse i.e., GS occur early in the course, and another type of seizure in combination with GS (epilepsy plus) occur late in the course in relatively older children. In a retrospective analysis of 100 cases, the authors of one study found a significantly older age and longer duration of disease in patients with gelastic epilepsy-plus seizures [8]. In a series of 60 cases of GS in HH, 51 patients began their seizures with brief GS [2]. In another study of 19 patients, GS were present in 16 out of 19 patients, and they were the first type of seizure to manifest in 14 patients [1].
Treatment of large HHs with refractory seizures and cognitive problems (as in the present case) involves surgical resection of the tumour at some stage along with multiple antiepileptic drugs and GnRH analogues for CPP. Various invasive and noninvasive surgical methods have been used because complications with open surgical procedures are often high. However, the main disadvantage of the nonsurgical procedure using the gamma knife is the delayed effect on seizure control, which usually takes 6 months or even longer. The other disadvantages of the gamma knife procedure include lower efficacy of seizure control (when compared to an open surgical approach) and radiation damage to surrounding vital structures (including the optic tract). In a prospective multicentric study of 60 HH patients treated with gamma knife, study of 60 patients, 27 patients treated with Gamma Knife surgery had at least 3 years follow up. Of these 27 patients, 59.2% had behavioural and cognitive improvement and all of these patients (59.2%) had significant improvement in seizure frequency [9]. Because we anticipated more complications with an open surgical procedure in the present case with the large tumour, gamma knife excision was performed. At the time of this report’s writing only 3 months have passed since the procedure, and it is too early to expect a significant response. Our follow-up plan is to observe the patient for 6–12 months for his response to the gamma knife surgery and repeat the brain MRI to look for reduction in tumour size. If there is no significant improvement in the seizures and the tumour is still large, a second gamma knife procedure may be planned along with higher doses of antiepileptics. We plan to continue the leuprolide depot injections until the patient is 11–12 years of age.
To conclude, a varied morphology of seizures is associated with HH, including atypical episodes of “trance-like state” as seen in our patient. The differential diagnoses entertained in any infant with seizures of uncertain aetiology and particularly early onset CPP should include HH. Children with the large sessile type of HH are likely to present with a combination of refractory seizures, cognitive impairment, developmental delay, and very early onset CPP.
Notes
Fig. 2.
Coronal T2-weighted image (A), sagittal T1-weighted image precontrast (B), and postcontrast (C) magnetic resonance images demonstrated a lobulated hypothalamic lesion (arrow) occupying the suprasellar cistern and displacing the pituitary stalk anteriorly (arrowhead). The lesion showed a slightly hyperintense signal relative to grey matter on T2 and an isointense signal on T1 with no significant enhancement on gadolinium administration.
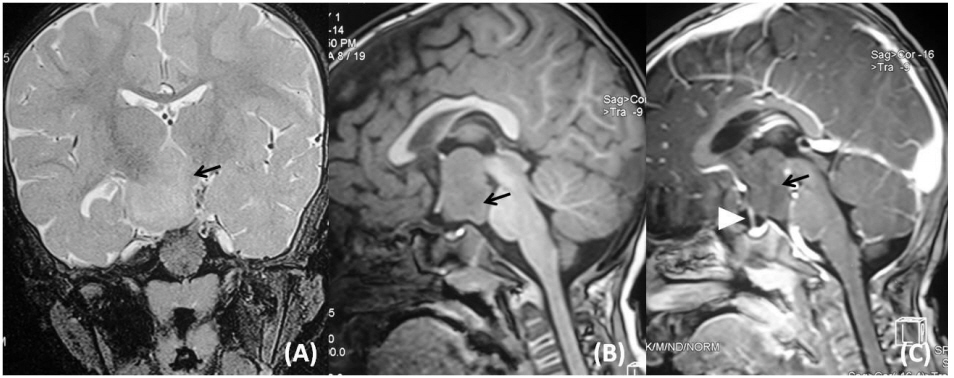
Table 1.
Summary of clinical and laboratory parameters at diagnosis and during the follow-up
| Parameter | At diagnosis 5 months | 1 Year | 1.5 Years | 2.5 Years | 4 Years | Last visit 5 Years & 4 months |
|---|---|---|---|---|---|---|
| Weight (kg) | 7.6 | 8.0 | 10.2 | 12 | 14.6 | 19 |
| Height (cm) | 62.5 | 71.6 | 78 | 89 | 93.5 | 103 |
| LH (IU/L)* | 0.98 | 0.82 | 1.16 | 0.523 | 0.464 | 0.448 |
| FSH (IU/L)* | 0.59 | 0.50 | 0.50 | 0.63 | - | 0.135 |
| Testosterone (nmol/L)* | 0.373 | 0.536 | 0.086 | 0.181 | 0.167 | |
| Testicular vol (mL) | 5 | 6 | 5 | 5 | 5 | |
| Pubic hair stage | 2 | 3 | 3 | 2 | 2 | |
| "Trance-like state" | Yes | - | - | - | - | - |
| Gelastic seizures | No | No | + | + | +++ | +++ |
| Injection leuprolide depot (mg 3 monthly) | 3.75 | 7.5 | 11.25 | 11.25 | 11.25 | 11.25 |
| Antiepileptics | Phenobarbitone Phenytoin | Phenytoin tapered off | Phenobarbitone | Levetiracetam Oxcarbazepine | Clobazam added | Levitracetam Oxcarbazepine Clobazam |
| Developmental milestones achieved | Social Smile Partial neck holding | Bisyllable speech | Independent walking | Cognition and behavioural issues | Running |
References
1. Mullatti N, Selway R, Nashef L, Elwes R, Honavar M, Chandler C, et al. The clinical spectrum of epilepsy in children and adults with hypothalamic hamartoma. Epilepsia 2003;44:1310–9.


2. Tassinari CA, Riguzzi P, Rizzi R. Gelastic seizures. Tuxhorn I, Hothausen Het al., editors. Paediatric epilepsy syndromes and their surgical treatment. London: John Libbey. 1997;pp 429–46.
3. Harrison VS, Oatman O, Kerrigan JF. Hypothalamic hamartoma with epilepsy: review of endocrine comorbidity. Epilepsia 2017;58 Suppl 2:50–9.


4. Delalande O, Fohlen M. Disconnecting surgical treatment of hypothalamic hamartoma in children and adults with refractory epilepsy and proposal of a new classification. Neurol Med Chir (Tokyo) 2003;43:61–8.


5. Le Marquand HS, Russell DS. A case of pubertas praecox in a boy associated with a tumour in the floor of the third ventricle. Berk Hosp Rep 1934-1935;3:31–61.
6. Kerrigan JF, Ng YT, Chung S, Rekate HL. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 2005;12:119–31.


7. Debeneix C, Bourgeois M, Trivin C, Sainte-Rose C, Brauner R. Hypothalamic hamartoma: comparison of clinical presentation and magnetic resonance images. Horm Res 2001;56:12–8.


- Related articles in APEM