|
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 23(3); 2018 > Article |
|
Abstract
This review describes the epidemiology of childhood diabetes in India. It focuses on the incidence and prevalence of type 1 diabetes and its complications and comorbid conditions. The review also covers data related to type 2 diabetes, glucose intolerance, and monogenic diabetes from India. A brief discussion regarding unique contributions from India to the world literature is included. The topics discussed include use of camel milk as adjuvant therapy in type 1 diabetes, relevance of the A1/A2 hypothesis, and comprehensive clinico-etiopathological classification of type 1 diabetes.
India, home to the largest population of children, also bears the heaviest burden of childhood morbidity in the world. India is known as one of the global diabetes epicenters of the global diabetes epidemic. The vast number of adults living with diabetes in the country means that most attention is focused on them, and on type 2 diabetes. Children with type 1 diabetes continue to fight for their rightful place under the sun [1]. Most epidemiologic studies and reviews of diabetes in India or South Asia focus on adults, while neglecting the significant burden of childhood diabetes. This review delves in to various facets of the epidemiology of paediatric diabetes in India.
The first attempt to assess the prevalence of insulin dependent diabetes in children was a population study carried out in 3 census zones of the northeastern part of Madras city (now named Chennai), in Tamil Nadu state. Cases were identified by data collection from hospitals, diabetes clinics, medical practitioners, chemists and druggists. Thirty children aged<15 years were identified, giving a prevalence of 0.261. The peak age at diagnosis was reported to be 12 years [2]. This was followed by a diabetes registry in the same city. The incidence of insulin dependent diabetes was found to be 12.6±11 and 9.6±4.7 per 100,000 boys and girls per year, over a 4-year period (1991–1994). The overall incidence was 10.5/100,000/yr, and peaked at age 10–12 years [3].
The neighbouring state of Karnataka instituted a multicentric registry which collected data over 13 years. This reported an incidence of 3.7/100,000 boys and 4.0/100,000 girls. However, the low figures may have been due to incomplete reporting from many parts of the large state [4].
Karnal district in Haryana state was the site of a hospital-based prevalence study. This district is served by a single endocrine centre which caters to the entire population. This study revealed an overall prevalence of 10.20/100,000 population with wide rural: urban and male: female gradients. The prevalence in Karnal city was 31.9/100,000, urban areas was 26.6/100,000, and in rural areas was 4.27/100,000. Men had a prevalence of 11.56/100,000, and in women, it was 8.6/100,000. In the 5 to 14 years age group, the prevalence was 24.22/100,000, while in the 0 to 6 years age group, it was 3.82/100,000. The total prevalence in the 0- to 14-year cohort was 18.27/100,000. The gender ratio was similar in toddlers and adolescents, and became skewed only in the >15 years age group [5]. The lower prevalence in rural areas suggests the lack of medical facilities, due to which many children with type 1 diabetes and undercurrent illness may not be diagnosed properly. The male: female discrepancy reflects gender discrimination, and the poor health care-seeking behavior exhibited by families for their daughters.
The government of India has conducted school surveys in Nainital (Uttarakhand state), Ratlam (Madhya Pradesh state), and Bhilwara (Rajasthan state) to assess the potential burden of childhood diabetes. According to the result, 1.467% of the 32,047 school children surveyed are suspected to have diabetes [6].
Type 1 diabetes does not always occur in isolation. It may be associated with various complications and comorbidities. An a analysis of 617 children aged ≤20 years with type 1 diabetes, who had undergone a minimum of 3 years of follow-up, was reported from Chennai. The authors detected retinopathy in 13.4% (background diabetic retinopathy 11.2%, proliferative diabetic retinopathy 1.9%, preproliferative 0.31%, maculopathy was seen in 13.3% of retinopathy cases), nephropathy in 7.1%, sensory neuropathy in 3.0%, ischaemic heart disease in 0.5% and peripheral vascular disease in 0.5% of participants. Duration of diabetes was positively associated with retinopathy, nephropathy and neuropathy. Glycosylated haemoglobin showed an association with retinopathy. Although the glycaemic control was suboptimal in this cohort, prevalence of vascular complications, especially macrovascular ones, seemed lower in Indian children as compared to what is reported from the west [7].
A large study conducted on 189 children with type 1 diabetes in Chandigarh revealed an 11.1% prevalence of celiac disease, which was diagnosed on the basis of serology and duodenal histology. In almost all cases, the diagnosis of diabetes preceded that of celiac disease by 5.18±4.75 years. Symptoms of celiac disease included short stature, anaemia, weight loss, diarrhea and abdominal pain [8].
Hypothyroidism is another auto immune endocrinopathy that commonly coexists with diabetes. In a single centre review of data at Karnal, 70 children aged <18 years (23 female, 47 male) with type 1 diabetes were screened for hypothyroidism. Four girls (17.4%) had a palpable goiter. Two girls were known cases of hypothyroidism, 5 girls were found to have overt hypothyroidism, and 2 boys were detected to have subclinical hypothyroidism. Thus the overall prevalence of hypothyroidism was 26.1% in girls and 4.2% in boys with type 1 diabetes [9].
Yet another endocrinopathy which is endemic to India is vitamin D deficiency. A study performed in Chandigarh assessed the plasma levels of 25-hydroxyvitamin D (25OHD), by high performance liquid chromatography, in 50 newly diagnosed children with diabetes. When compared with age matched healthy controls (6–12 years age), the mean levels of vitamin D were found to be significantly lower (20.02±10.63 ng/mL vs. 26.16±12.28 ng/mL, P=0.009). Vitamin D deficiency (25OHD<20 ng/mL) was detected in 58% children with diabetes as compared to 32% controls. When deficiency and insufficiency (25OHD<30 ng/mL) were collated, the prevalence rose to 86% in type 1 diabetes and 76% in healthy children [10].
India is a pay-from pocket market, where health insurance coverage is sparse [11]. A study in Chennai aimed to assess the economic burden of type 1 diabetes on the families of youth and adolescents with type 1 diabetes. A median annual expenditure on diabetes of Indian rupees 13,980 (USD 310) was reported. The median percentage of income spend on diabetes was 59% in low socioeconomic families, 32% in the middle socioeconomic stratum, and 12% in the high income group. The overall average was 22%. Indoor hospitalization was associated with higher economic burden (23% of family income) as compared to outdoor management (16% of income) [12].
Not all childhood diabetes is type 1, and Indian researchers have been cognizant of this fact [13]. Perhaps the earliest report of type 2 diabetes in children was from Madras. A case series of 18 children with insidious onset of diabetes at age ≤15 years, response to oral glucose lowering drugs (preserved C peptide levels [≥0.6 pmol/mL]) and absence of decarboxylase 65 (glutamic acid decarboxylase 65) antibodies was reported [14].
Type 2 diabetes in children is gradually being reported across the country, through the prevalence is not as high as in Asian countries such as Japan. At referral centres such as Lucknow and Chennai, the proportion of children with type 2 diabetes is reported as 12% and 26.7% respectively. This, however, may be due to a referral bias [15]. Various hospital and clinic based registries, reviewed by Chowdhury and Ghosh, suggest that the percentage of type 1 diabetes in children is showing a declining trend. This implies that the prevalence of type 2 diabetes in children is rising [15].
A large project (n=1,519), which studied children aged 6–11 years in Chennai found an overall prevalence of 3%–7% of glucose intolerance (4.2% in girls, 3.2% in boys; P<0.001). The prevalence of dysglycaemia, as assessed by oral glucose tolerance test, was 12.7% in girls with abdominal obesity. Upon multivariate analysis, family history of diabetes was found to have significant association with glucose intolerance in girls (odds ratio [OR], 4.11; 95% confidence interval [CI], 1.28–13.22; P=0.018). Homeostasis model assessment-insulin resistance (HOMA-IR) was significantly associated with glucose intolerance in both boys (OR, 5.19; 95% CI, 1.54–17.44; P=0.008) and girls (OR, 11.22; 95% CI, 4.19–30.05; P<0.001) [16]. The high prevalence of glucose intolerance in this young age group is cause for worry
Monogenic diabetes mellitus is also documented and studied in India. A descriptive cohort study from Chennai (1999–2007: retrospective; 2008–2012: prospective design) included 40 infants with onset of diabetes at age ≤1 year. These constituted 8% of all children with onset of diabetes ≤12 years. Low birth weight was found in 63% of all infants with onset of diabetes ≤6 months of age, and 30% with onset at 6–12 months age. Monogenic forms of diabetes were found in 84.5% of infants with onset ≤6 months age, and 55.0% of those with onset at 6–12 months. The commonest form was Wolcott Rallison syndrome, while KCNJ11 and ABCC8 mutations were also reported. Delayed diagnosis, recurrent hospitalization, developmental delay, and high childhood mortality (32.5% at 12.5-year follow-up) were noted by the authors [17].
A recent publication from the same city (Chennai) described the clinical presentation and molecular characterization of 33 Indian children with onset of diabetes below 12 months of age. Twelve mutations were identified, including those in ABCC8 (7/12), KCNJ11 (3/12), and INS (2/12). Novel mutations that were discovered included Asp 212Tyr mutation in ABCC8, Val67Met in Berardinelli Seip Syndrome and Leu1Arg in Fanconi Bickel syndrome [18]. This study highlights the diversity of monogenic diabetes in India, and the need to screen for it carefully.
Indian have a higher percentage of body fat and visceral fat, when compared with Caucasians of a similar body mass index. This has led to the characterization of a "thin fat" Indian diabetes phenotype. This phenotype is present from birth, and may be worsened by accelerated childhood growth. It is speculated that this pathophysiological state contributes to the earlier development of type 2 diabetes and metabolic syndrome in children in India [19]. Such understanding will facilitate further growth in diabetology.
This hypothesis is supported by findings that offspring of mothers with diabetes have thicker skinfolds, and higher glucose levels, insulin concentrations, HOMA-IR and systolic blood pressure, as compared to controls [20]. Thus, gestational diabetes has now been identified as a target for prevention of diabetes in offspring [21].
Yet another hypothesis, which attempts to explain the increasing incidence of type 1 diabetes in India, is the A1/A2 milk hypothesis [22]. Most cow milk protein is found in the form of casein and whey. One form of casein, beta casein, can be present as any of 12 genetic variants. The most common variant are the A1 and A2 variant. Proteolysis of the A1 variant in the gastro intestinal tract generates an immunogenic bioactive peptide called beta casomorphin 7 (BCM7), which is absorbed by the immature gut of infants.
In recent decades, Indian dairy production has shifted from using indigenous cow breeds (Zebu cows) to exotic breeds (taurine) and buffalos. While indigenous cow milk beta casein is A2/A2 in nature, buffalo milk contains A1/A2 beta casein, and exotic breeds have A1/A1 alleles. It is hypothesized that exposure to immunogenic A1/A1 milk may be linked with the rising incidence of type 1 diabetes in India. An interesting observation from the desert state of Rajasthan is the use of camel milk as adjuvant therapy in type 1 diabetes [23].
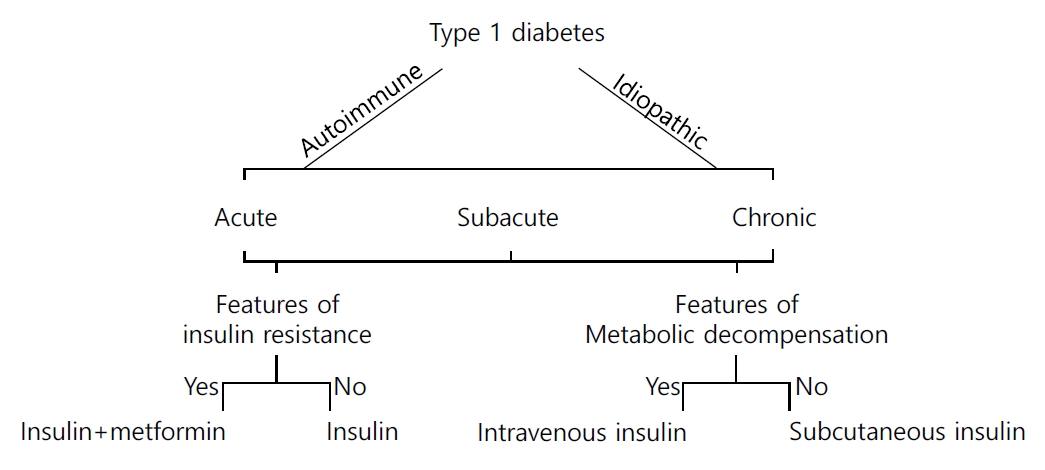
The classification of diabetes is a dynamic field of characterized by debate and discussion. There are significant differences, for example, between Japanese and American and international classifications of the condition [24]. The Japan Diabetes Society classifies type 1 diabetes into fulminant, acute onset or slowly progressive, based upon clinical presentation and progression. Indian endocrinologists have begun recognizing these variants (personal communication: Prof Rakesh Sahay, Hyderabad), but continue to follow western classification systems.
Recently, Indian experts have proposed a clinico-etiological taxonomic rubric for type 1 diabetes. This is based upon three variables: etiology, mode of onset and clinical presentation [24]. This system of classification includes both western and Japanese thought, is compatible with modern understanding of pathophysiology, clinically oriented, and lends support to (Table 1, Fig. 1) therapeutic decision-making. The classification system can be used in settings where antibody estimation is not possible, as it includes a glucophenotypic basis of description as well [25].
Data from India reveals a significant prevalence of type 1 diabetes (over 10/100,000 population), with certain urban pockets reporting over 30/100,000 population). At the same time, the burden of glucose intolerance (associated with abdominal obesity) and type 2 diabetes is increasing in children. Novel explanations such as the A1/A2 milk hypothesis and “thin fat” Indian phenotype help explain the trend of increase in type 1 and type 2 diabetes in children. Newer clinical classification of diabetes provide a unified overview of the condition, through a clinico-etiopathologic prism.
Table 1.
Classification of type 1 diabetes proposed from India
References
1. Kumar KM, Azad K, Zabeen B, Kalra S. Type 1 diabetes in children: Fighting for a place under the sun. Indian J Endocrinol Metab 2012;16 Suppl 1:S1–3.

2. Ramachandran A, Snehalatha C, Abdul Khader OM, Joseph TA, Viswanathan M. Prevalence of childhood diabetes in an urban population in south India. Diabetes Res Clin Pract 1992;17:227–31.


3. Ramachandran A, Snehalatha C, Krishnaswamy CV. Incidence of IDDM in children in urban population in southern India. Madras IDDM Registry Group Madras, South India. Diabetes Res Clin Pract 1996;34:79–82.


4. Kumar P, Krishna P, Reddy SC, Gurappa M, Aravind SR, Munichoodappa C. Incidence of type 1 diabetes mellitus and associated complications among children and young adults: results from Karnataka Diabetes Registry 1995-2008. J Indian Med Assoc 2008;106:708–11.

5. Kalra S, Kalra B, Sharma A. Prevalence of type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetol Metab Syndr 2010;2:14.



6. Kumar KM. Incidence trends for childhood type 1 diabetes in India. Indian J Endocrinol Metab 2015;19(Suppl 1):S34–5.



7. Ramachandran A, Snehalatha C, Sasikala R, Satyavani K, Vijay V. Vascular complications in young Asian Indian patients with type 1 diabetes mellitus. Diabetes Res Clin Pract 2000;48:51–6.


8. Bhadada SK, Kochhar R, Bhansali A, Dutta U, Kumar PR, Poornachandra KS, et al. Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in north India. J Gastroenterol Hepatol 2011;26:378–81.


9. Kalra S, Kalra B, Chatley G. Prevalence of hypothyroidism in pediatric type 1 diabetes mellitus in Haryana, Northern India. Thyroid Res Pract 2012;9:12–4.

10. Borkar VV, Verma S, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes 2010;11:345–50.


11. Sahay R, Baruah MP, Kalra S. Health economics in India: the case of diabetes mellitus. Indian J Endocrinol Metab 2014;18:135–7.



12. Shobhana R, Rama Rao P, Lavanya A, Williams R, Padma C, Vijay V, et al. Costs incurred by families having Type 1 diabetes in a developing country--a study from Southern India. Diabetes Res Clin Pract 2002;55:45–8.


13. Narayan KM, Fagot-Campagna A, Imperatore G. Type 2 diabetes in children: a problem lurking for India? Indian Pediatr 2001;38:701–4.

14. Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Type 2 diabetes in Asian-Indian urban children. Diabetes Care 2003;26:1022–5.


15. Chowdhury S, Ghosh S. Epidemiology of childhood diabetes. Das AK, Kalra Set al., editors. CDiC textbook of pediatric diabetes. New Delhi (India): Jaypee. 2018;pp 8–20.
16. Ranjani H, Sonya J, Anjana RM, Mohan V. Prevalence of glucose intolerance among children and adolescents in urban South India (ORANGE-2). Diabetes Technol Ther 2013;15:13–9.


17. Varadarajan P, Sangaralingam T, Senniappan S, Jahnavi S, Radha V, Mohan V. Clinical profile and outcome of infantile onset diabetes mellitus in southern India. Indian Pediatr 2013;50:759–63.


18. Jahnavi S, Poovazhagi V, Mohan V, Bodhini D, Raghupathy P, Amutha A, et al. Clinical and molecular characterization of neonatal diabetes and monogenic syndromic diabetes in Asian Indian children. Clin Genet 2013;83:439–45.


19. Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004;134:205–10.



20. Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010;33:402–4.


21. Kalra S, Gupta Y, Kumar A. Prevention of gestational diabetes mellitus (GDM). J Pak Med Assoc 2016;66(9 Suppl 1):S107–9.

22. Sodhi M, Mukesh M, Kataria RS, Mishra BP, Joshii BK. Milk proteins and human health: A1/A2 milk hypothesis. Indian J Endocrinol Metab 2012;16:856.



23. Agarwal RP, Swami SC, Beniwal R, Kochar DK, Sahani MS, Tuteja FC, et al. Effect of camel milk on glycemic control, risk factors and diabetes quality of life in type-1 diabetes: a randomized prospective controlled study. J Camel Pract Res 2003;10:45–50.
24. Kalra S, Gupta Y, Khandelwal D. Classification of type 1 diabetes (T1DM). J Pak Med Assoc 2018;68:318–21.