The relationship between subclinical hypothyroidism and serum levels of uric acid and creatinine in children aged 2–14 years
Article information
Abstract
Purpose
Hypothyroidism is a clinical syndrome that can lead to elevated levels of serum creatinine and uric acid by causing impaired renal function. Although many studies have been carried out on the relationship between overt hypothyroidism and renal function, few studies have been conducted on subclinical hypothyroidism and renal function, especially in pediatric patients. For this reason, we studied this issue in children, so as to provide a background for more useful research and future education.
Methods
This case-control study was performed on 107 children aged 2–14 years, 56 children with subclinical hypothyroidism in the case group, and 51 healthy children in the control group presenting to Ayatollah Mousavi Hospital in Zanjan and private clinics of Zanjan city. Thyroid stimulating hormone, triiodothyronine, thyroxine, creatinine, and uric acid were measured in both groups of children after obtaining the necessary criteria for entering the study.
Results
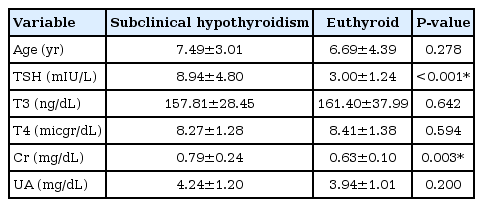
Compared to the control group, subjects with subclinical hypothyroidism had higher levels of creatinine (P=0.003), while serum uric acid levels in subclinical hypothyroid children was not significantly different from those in the control group (P=0.200).
Conclusions
In subclinical hypothyroidism in children, creatinine was higher than in euthyroid patients, but uric acid was not different.
Introduction
The interactions between thyroid gland and renal functions are known for years [1,2]. Thyroid dysfunction can affect renal physiology and development, and on the other hand, kidney disorders can confluence thyroid function [3]. Hypothyroidism, in turn, slows down all metabolic processes. One of these metabolic pathways is the purine metabolism pathway. Hypothyroidism by influencing this pathway causes changes in uric acid levels [4]. In hypothyroidism, due to decreased cardiac output, increased peripheral vascular resistance, vasoconstriction in the renal vasculature, reduced renal response to vasodilators, and decreased expression of renal vasodilators, such as vascular endothelial growth factor and insulin like growth factor 1, renal blood flow (RBF) is reduced. Glomerular filtration rate (GFR) also diminishes due to reduced sensitivity to beta-adrenergic stimuli and decreased renin secretion. Decreasing GFR and RBF along with rhabdomyolysis and myopathy induced by hypothyroidism increase serum creatinine [5,6]. Hypothyroidism is a clinical syndrome that is divided into overt and subclinical forms. To date, numerous studies have confirmed the association between serum levels of uric acid and creatinine with overt hypothyroidism [4,7-10]. However, data on the relationship between subclinical thyroid disorders and serum uric acid and creatinine level are very limited. On the other hand, all of these studies have been performed on adolescents with hypothyroidism, and less study has been conducted in pediatric patients. A number of authors in a study in 1999 investigated the effects of long-acting hypothyroidism on renal function on 5 children, and acknowledged that kidney impairment that occurs in children with hypothyroidism is much more severe than in adults with hypothyroidism [11]. This study was therefore planned to evaluate the changes in biochemical markers of renal function in subclinical hypothyroid pediatric subjects.
Materials and methods
1. Study design
This case-control study was performed on 107 children aged 2–14 years old (56 children with subclinical hypothyroidism in the case group and 51 healthy children in the control group) who referred to Ayatollah Mousavi Hospital and private clinics in Zanjan in November 2016 to September 2017. This study was approved by the ethics committee of Zanjan University of Medical Sciences (ZUMS.REC.1395.159).
Initially, for children referred to these centers, after the clinician's suspicion for thyroid problems according to ectodermal (poor growth, dull facies, dry scaly skin, sparse brittle hair, diminished sweating); circulatory (sinus bradycardia, cold extremities, cold intolerance, pallor, ECG changes: low-voltage QRS complex); neuromuscular (muscle weakness, hypotonia [constipation, potbelly], umbilical hernia, myxedema coma, pseudo hypertrophy of muscles, myalgia, physical and mental lethargy, developmental delay, delayed relaxation of reflexes, paresthesias, cerebellar ataxia); skeletal (delayed bone age, epiphyseal dysgenesis) and metabolic (serous effusions [pleural, pericardial, ascites], hoarse voice [cry], weight gain, menstrual irregularity, arthralgia, elevated creatine kinase, macrocytosis [anemia], hypercholesterolemia, hyperprolactinemia) symptoms and signs of hypothyroidism, the thyroid-stimulating hormone (TSH) test was requested. If the TSH level was higher than upper limit of normal by age, they were also requested to undergo triiodothyronine (T3) and thyroxine (T4) hormonal tests. The diagnosis of subclinical hypothyroidism was performed by the physician by interpreting the results of tests based on increased TSH levels based on the age and normal range of T3 and T4. Patients who were under treatment with levothyroxine and steroids were excluded from the study. Other exclusion criteria were: having proven hypertension, diabetes mellitus, liver disorders, renal disorders, cardiovascular disorders and patients who had malignancy or were under chemotherapy or radiation therapy. Both groups of children whose body mass index was in the 5th to 85th percentile (according to the Center for Disease Control and Prevention [CDC] classification) were included in the study.
After the demonstration of subclinical hypothyroidism, considering the inclusion and exclusion criteria and the signing of written consent by parents, these children were selected as the case group for entering the study. The serum levels of TSH, T3, T4, uric acid and creatinine were again requested to be measured at a single laboratory (Ayatollah Mousavi Hospital Lab), after 8 hours of fasting. By taking 3 mL of venous blood sample, the serum levels of uric acid and creatinine were measured by biochemical methods with Dirui auto-analyzer while serum levels of TSH, T3, and T4 were measured by enzyme-linked immunosorbent assay reader. In the control group, the healthy children were recruited according to inclusion and exclusion criteria after their parents signed a consent form. As with the children with hypothyroidism, serum levels of TSH, T3, T4, uric acid, and creatinine were measured in the laboratory of the same center.
2. Statistical analysis
In this study, data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) and reported as mean±standard deviation. The distribution of quantitative data was assessed by using the Kolmogorov-Smirnov test. To compare continuous quantitative data with normal distribution (age, T3, T4, and uric acid) between case and control groups, independent t-test and to compare the two data of TSH and Cr with abnormal distribution between the 2 groups, the Mann-Whitney test was used. Comparison of only qualitative data (sex) was performed using chi-square test. By using Pearson and Spearman correlation tests, the relationship between continuous variables was tested. Box and Whisker charts were drawn. By using the S-Data software version 12, quantile regression was performed for creatinine and uric acid variables. A P<0.05 was considered to be statistically significant.
Results
In this study, in case group, 48.2% were female and 51.8% were male and in control group, 42.1% were female and 57.9% were male. The mean age of children in the case group was 7.49±3.01 years and in the control group was 6.69±4.39 years. The 2 groups were comparable in terms of age (P=0.278) and sex (P=0.629) and there was no significant difference between them. The values of thyroid hormones and biochemical markers indicating renal function in both case and control groups are summarized in Table 1. As shown in Table 1, TSH and Cr values in children with subclinical hypothyroidism were significantly higher than those in the control group (P<0.05). The levels of uric acid, T3 and T4 did not differ significantly between the 2 groups.
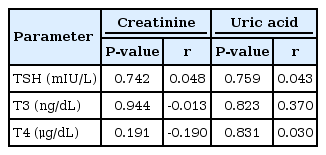
In this study, we also investigated the relationship between renal function markers and thyroid function tests. According to the results, TSH, T3, and T4 had no significant correlation with any of the parameters of renal function (Table 2).
We also plotted the Box and Whisker charts in order to compare the differences between creatinine and uric acid in the case and control groups (Fig. 1).
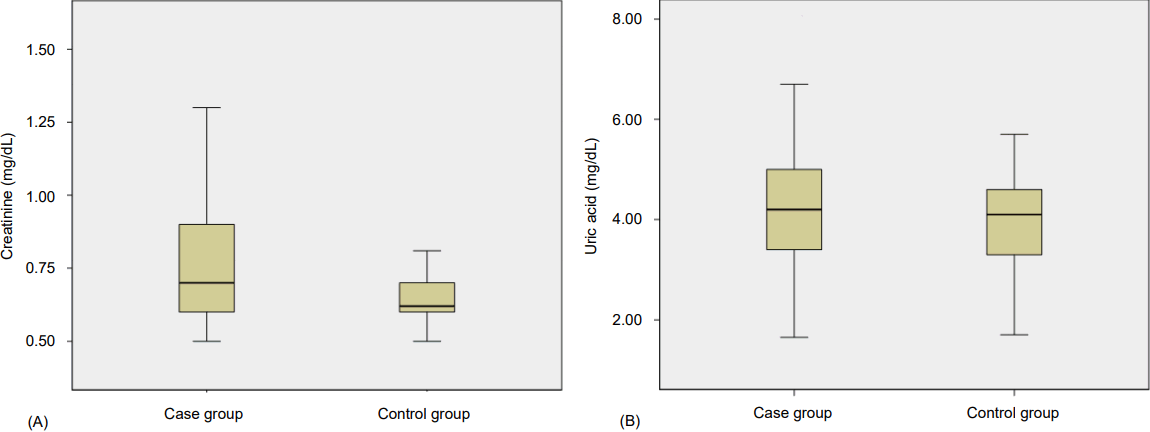
(A) Box and whisker plot showing comparison of creatinine between case and control group. The boxes contain 50% of values between the 25th and 75th percentiles; the whiskers indicate the highest and lowest values. The horizontal line inside each box represents the median. (B) Box and whisker plot showing comparison of uric acid between case and control group. The boxes contain 50% of values between the 25th and 75th percentiles; the whiskers indicate the highest and lowest values. The horizontal line inside each box represents the median.
Moreover, multiple regression results based on quarter showed that subclinical hypothyroidism was not associated with uric acid levels in any of the quartiles (Table 3).
The results of quantile regression based on the quarter showed that there was a significant relationship between the study group and the creatinine level only in those who were placed in the above quartile of creatinine. According to code 1 for case group and 2 for control group and negative regression coefficients, subclinical hypothyroidism is correlated positively with creatinine level in high quartiles of creatinine (Table 4).
Discussion
To date, very limited studies have examined the relationship between subclinical hypothyroidism and biochemical markers that indicate renal function [9,12-14]. All of these studies have been conducted in adults. By searching in MEDLINE, we could not find any study about this subject in pediatric patients and the recent study seems to be the first study to examine the relationship between subclinical hypothyroidism and biochemical markers of kidney function in children. In this case-control study, we recruited 56 children with subclinical hypothyroidism in the case group and 51 healthy children in the control group and finally we concluded that serum creatinine was significantly higher in the case group than the control group. The results showed that, although the amount of uric acid in the subclinical hypothyroid children group was slightly higher than healthy children, this difference was not statistically significant. In line with our study, Tayal et al. [9], in a case-control study that includes 98 patients in the subclinical hypothyroidism group, 89 in the overt hypothyroidism group and 187 in the control group, stated that serum creatinine level was significantly higher in subjects with subclinical and overt hypothyroidism than in control group (P<0.01). In line with the results of our study, they did not show a significant increase in serum uric acid in patients with subclinical hypothyroidism in comparison to the control group. In a similar study, Kaur et al. [12] examined the relationship between overt and subclinical hypothyroidism and renal function. In their research, 100 subjects in the control group, 50 in the overt hypothyroidism group and 50 in the subclinical hypothyroid group were studied. All participants in the study were between 20–70 years of age. At the end, they also concluded that subclinical hypothyroidism significantly increased serum creatinine (P<0.001), while they did not show significant increases in serum uric acid. Verhelst et al. [13] in a study that was designed to find out the relationship between serum creatinine and other Guanidino compounds in patients with thyroid dysfunction, concluded that in patients with subclinical hypothyroidism, serum creatinine levels increased. In their study, uric acid serum was not measured. Liang et al. [14], in a study that was conducted on 356 subclinical hypothyroid patients in the case group and 331 in the control group, concluded that serum uric acid in subclinical hypothyroid patients significantly increased. In their study, serum creatinine was not studied. Regarding serum uric acid levels in subclinical hypothyroid patients, the results of our study were in line with most studies and contrary to the results of Liang study. For this purpose, one of the important tasks of thyroid hormones (T3 and T4) is to regulate various metabolic pathways in the body. One of these metabolic pathways that can be affected by thyroid hormones, is purine metabolism pathway. This metabolic pathway is one of the most effective cycles in the production of uric acid. Increasing or decreasing T3 and T4 leads to changes in the purine metabolism cycle and ultimately alteration in production of uric acid [4,15,16]. Whereas in subclinical hypothyroidism, the blood levels of T3 and T4 hormones are maintained within the normal range, and it is therefore entirely reasonable that the level of uric acid remains in the natural range. In addition, several authors in their research on hypothyroidism have investigated the serum levels of uric acid in patients with overt and sub-clinical hypothyroidism simultaneously [9,10,12]. Tayal et al. [9] concluded that although subclinical hypothyroidism does not change serum levels of uric acid, but in patients with clinical hypothyroidism, the serum levels of uric acid clearly increase. In their study, TSH levels were reported in the clinical hypothyroid group as 41.46±1.01 and TSH levels in subclinical hypothyroid patients was 11.9±0.50. Kaur et al. [12] obtained similar results with Tayal regarding serum uric acid levels in patients with overt and subclinical hypothyroidism. In their study, TSH levels in the clinical and subclinical hypothyroid patients were 46.06±58.06 and 7.12±1.32, respectively. Arora et al. [10], demonstrated that in patients with overt hypothyroidism, serum uric acid levels were significantly higher than in the control group. The level of patient’s TSH in this study was 36.44±15.48. So it seems that serum uric acid increases at high levels of TSH. However, in patients with subclinical hypothyroidism, the mean TSH was 8.94±4.80 in the present study.
It should be noted that since serum uric acid can be affected by diet [17], part of the difference between these studies can be attributed to differences in dietary habits of participants in the study. In our study, control and case groups were not matched through diet. On the other hand, thresholds for TSH levels that increase serum uric acid levels in subclinical hypothyroidism may be different in different races.
In justifying the findings of recent research on serum creatinine levels, it can be stated that increasing serum creatinine levels in patients with subclinical hypothyroidism is quite reasonable. Various studies have demonstrated that increased levels of TSH, both in hypothyroidism and in subclinical hypothyroidism, are associated with a decrease in GFR. Following a reduction in GFR, renal plasma flow decreases and consequently serum creatinine increases.18-20]. In this study, we could not find any relationship between renal function factors and thyroid parameters. Although few studies have investigated this issue, but studies by Tayal et al. [9] and Kaur et al. [12] confirm our results. They also failed to achieve a relationship between the parameters of renal function and thyroid tests in adult patients with subclinical hypothyroidism. Due to the high similarity in the function of thyroid hormones in children and adults in many cases, these findings can be generalized to children.
At the end, it should be noted that since subclinical hypothyroidism is a relatively common condition in children [21], all of its aspects, especially its association with renal function, physicians should be trained on it. This is especially important when it has been shown that abnormal kidney function in patients with hypothyroidism is reversible [9,22]. In addition, a study by Elgadi et al. [23] has shown that in the long term, in children with hypothyroidism treated with levothyroxine, decline in renal function will return 1–5 years after the onset of treatment. Considering the results of this study and the above mentioned issues, it seems that monitoring of renal function in children with subclinical hypothyroidism should be considered. Moreover, in the presence of creatinine changes, subclinical hypothyroidism can be considered as one of the causes that increases this factor. It is suggested that future studies with a larger sample size be done to confirm the results of recent research on children with subclinical hypothyroidism.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.