 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 22(3); 2017 > Article |
|
Abstract
Purpose
Methods
Results
Fig. 1.
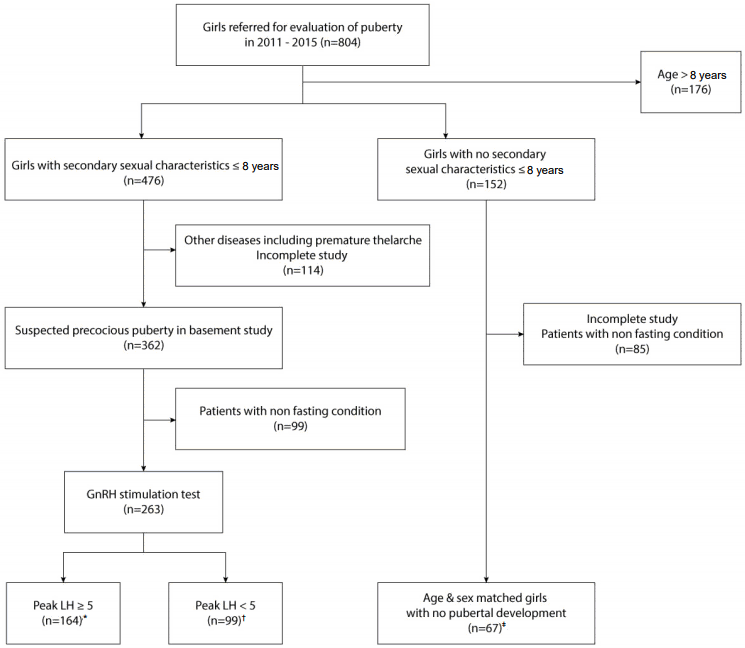
Table 1.
| Characteristic | CPP (n=164) | Non-CPP (n=99) | Control (n=67) | P-value* | P-value† | P-value‡ | Overall P-value§ |
|---|---|---|---|---|---|---|---|
| CA (yr) | 8.3±0.6 | 8.1±0.8 | 7.9±0.6 | 0.001 | 0.077 | 0.056 | 0.001 |
| BA (yr) | 9.7±0.9 | 9.2±1.0 | 8.2±0.9 | 0.001 | 0.001 | 0.003 | 0.001 |
| BA–CA (yr) | 1.3±0.7 | 1.1±0.7 | 0.0±1.5 | 0.001 | 0.001 | 0.117 | 0.001 |
| Ht. SDS | 0.9±1.1 | 0.8±1.0 | 0.5±0.9 | 0.011 | 0.063 | 0.999 | 0.012 |
| Wt. SDS | 0.7±1.3 | 0.9±1.3 | 0.5±0.9 | 0.714 | 0.142 | 0.782 | 0.138 |
| BMI SDS | 0.3±1.1 | 0.9±1.3 | 0.4±0.9 | 0.999 | 0.633 | 0.237 | 0.195 |
| SHBG (nmol/L) | 80.2±36.7 | 76.5±34.7 | 83.3±31.5 | 0.999 | 0.671 | 0.999 | 0.460 |
| LH0 (IU/L) | 1.1±1.2 | 0.5±0.4 | 0.3±0.3 | 0.001 | 0.615 | 0.001 | 0.001 |
| Peak LH (IU/L) | 12.9±9.5 | 3.2±1.0 | - | - | - | 0.003 | 0.001 |
| Peak LH/LH0 | 23.3±22.7 | 9.3±5.8 | - | - | - | 0.003 | 0.001 |
| E2 (pg/mL) | 12.0±12.5 | 9.5±9.0 | 5.0±0.3 | 0.001 | 0.016 | 0.162 | 0.001 |
| FEI (pmol/L) | 17.8±18.3 | 15.3±17.1 | 7.0±2.7 | 0.001 | 0.004 | 0.675 | 0.001 |
| IGF-1 (ug/L) | 271.6±91.4 | 238.9±70.9 | 220.6±64.3 | 0.001 | 0.463 | 0.005 | 0.001 |
| AST (IU/L) | 27.3±8.9 | 28.6±10.3 | 27.1±5.1 | 0.999 | 0.883 | 0.713 | 0.433 |
| ALT (IU/L) | 14.8±5.7 | 17.5±12.1 | 14.8±6.7 | 0.999 | 0.112 | 0.037 | 0.028 |
| Total cholesterol (mg/dL) | 168.5±28.7 | 164.7±27.2 | 169.4±23.0 | 0.999 | 0.831 | 0.842 | 0.457 |
| Triglycerides (mg/dL) | 116.0±68.6 | 109.5±61.6 | 111.4±58.1 | 0.999 | 0.999 | 0.999 | 0.718 |
| LDL-cholesterol (mg/dL) | 94.6±27.1 | 93.5±26.6 | 95.9±21.8 | 0.999 | 0.999 | 0.999 | 0.850 |
| HDL-cholesterol (mg/dL) | 51.2±10.1 | 50.4±9.9 | 51.8±8.5 | 0.999 | 0.999 | 0.999 | 0.637 |
| Fasting plasma glucose (mg/dL) | 94.7±11.1 | 96.1±11.9 | 91.8±8.1 | 0.204 | 0.035 | 0.864 | 0.040 |
| Insulin (μU/mL) | 23.5±22.9 | 22.5±26.5 | 12.0±6.8 | 0.001 | 0.008 | 0.999 | 0.001 |
| HOMA-IR | 5.69±5.85 | 5.68±7.37 | 2.78±1.72 | 0.002 | 0.005 | 0.999 | 0.002 |
| QUICKI | 0.32±0.04 | 0.33±0.05 | 0.34±0.04 | 0.003 | 0.194 | 0.390 | 0.004 |
Values are presented as mean±standard deviation.
CPP, central precocious puberty; CA, chronological age; BA, bone age; SDS, standard deviation scores; Ht., height; Wt., weight; BMI, body mass index; SHBG, sex hormone binding globulin; LH, luteinizing hormone; E2, estradiol; FEI, free estradiol index; IGF-1, insulin-like growth factor-1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index
Table 2.
| Characteristic | BMI<50th %ile (n=87) | 50th %ile≤BMI<85th %ile (n=57) | BMI≥85th %ile (n=20) | P-value* | P for trend† |
|---|---|---|---|---|---|
| CA (yr) | 8.3±0.6 | 8.4±0.6 | 8.2±0.7 | 0.482 | 0.960 |
| BA (yr) | 9.6±0.9 | 9.6±0.9 | 10.2±1.0 | 0.018 | 0.018 |
| BA–CA (yr) | 1.2±0.7 | 1.2±0.7 | 2.0±0.9 | 0.001 | 0.001 |
| SHBG (nmol/L) | 98.6±34.2 | 66.0±27.2 | 40.7±17.0 | 0.001 | 0.001 |
| LH0 (IU/L) | 1.1±1.3 | 1.1±1.3 | 1.1±0.8 | 0.952 | 0.842 |
| Peak LH (IU/L) | 12.7±9.7 | 13.3±9.2 | 12.5±9.9 | 0.924 | 0.953 |
| E2 (pg/mL) | 13.2±14.1 | 10.3±10.3 | 11.2±9.6 | 0.367 | 0.259 |
| FEI (pmol/L) | 15.0±16.7 | 18.1±18.7 | 29.5±20.1 | 0.007 | 0.004 |
| IGF-1 (ug/L) | 278.4±94.0 | 251.5±87.4 | 298.9±83.5 | 0.081 | 0.950 |
| AST (IU/L) | 26.4±5.7 | 29.6±12.5 | 24.3±6.8 | 0.032 | 0.887 |
| ALT (IU/L) | 12.8±3.4 | 16.1±6.0 | 19.8±8.0 | 0.001 | 0.001 |
| Total cholesterol (mg/dL) | 165.6±25.7 | 174.0±33.2 | 165.2±26.2 | 0.198 | 0.457 |
| Triglycerides (mg/dL) | 108.3±72.8 | 122.4±66.3 | 130.9±53.4 | 0.284 | 0.117 |
| LDL-cholesterol (mg/dL) | 92.8±27.3 | 98.3±26.6 | 92.4±27.6 | 0.457 | 0.630 |
| HDL-cholesterol (mg/dL) | 52.0±10.5 | 51.2±9.6 | 46.9±8.7 | 0.139 | 0.081 |
| Fasting plasma glucose (mg/dL) | 94.3±11.6 | 94.4±11.2 | 96.9±8.1 | 0.647 | 0.461 |
| Insulin (μU/mL) | 17.2±13.9 | 23.2±22.8 | 51.9±32.8 | 0.001 | 0.001 |
| HOMA-IR | 4.15±3.87 | 5.60±5.79 | 12.60±8.14 | 0.001 | 0.001 |
| QUICKI | 0.33±0.04 | 0.32±0.04 | 0.28±0.02 | 0.001 | 0.001 |
Values are presented as mean±standard deviation.
BMI, body mass index; CPP, central precocious puberty; CA, chronological age; BA, bone age; SHBG, sex hormone binding globulin; LH, luteinizing hormone; E2, estradiol; FEI, free estradiol index; IGF-1, insulin-like growth factor-1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index.
Table 3.
Insulin resistance is positively correlated with BA advancement, BMI SDS, FEI, IGF-1, and triglycerides and was negatively correlated with SHBG.
HOMA-IR, homeostasis model assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index; BA, bone age; CA, chronological age; SDS, standard deviation scores; BMI, body mass index; SHBG, sex hormone binding globulin; LH, luteinizing hormone; FEI, free estradiol index; IGF-1, insulin-like growth factor-1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, lowdensity lipoprotein.
Table 4.
When insulin resistance increased, the odds for BA advancement increased. Model 1 is a multiple logistic regression adjusted for chronological age. Adjusted OR for chronological age and BMI represented in model 2.
HOMA-IR, homeostasis model assessment-insulin resistance; QUICKI, quantitative insulin sensitivity check index; SE β, standard error of β coefficient; OR, odds ratio; CI, confidence interval; model 1, adjusted for chronological age (CA); model 2, adjusted for CA and body mass index (BMI).
References
- TOOLS
- Related articles in APEM







