Insulin and glucagon levels of umbilical cord blood in appropriate for gestational age - preterm infants with or without postnatal hypoglycemia
Article information
Abstract
Purpose
To determine whether serum insulin and glucagon levels of umbilical cord blood correlate with subsequent postnatal hypoglycemia in appropriate for gestational age (AGA) – preterm infants at different gestational ages (GAs).
Methods
The serum insulin and glucagon levels of umbilical cord blood were measured using magnetic bead based multiplex immunoassay in 69 AGA - premature infants, stratified according to GA: GA 23–30 weeks, early preterm (EP, n=31); GA 31–34 weeks, late preterm (LP, n=38). Postnatal hypoglycemia was defined as a capillary glucose level <40 mg/dL within the first 60 minutes of life, regardless of GA.
Results
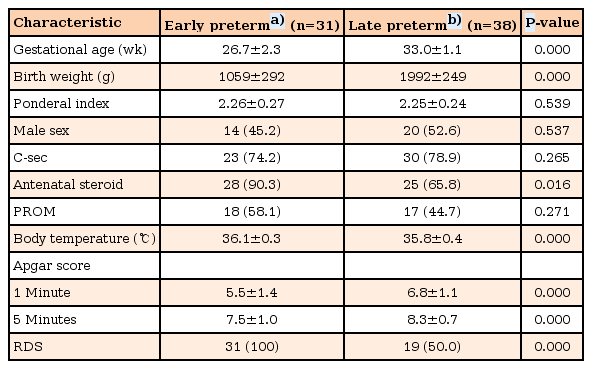
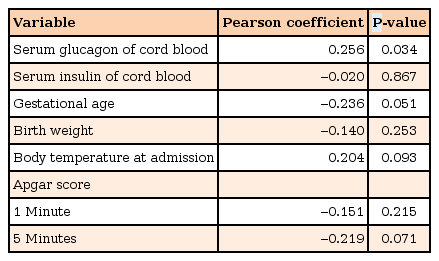
The capillary glucose concentration in EP infants (65.5±21.2 mg/dL) was significantly higher than that of LP infants (55.9±17.3 mg/dL) (P=0.043). The serum glucagon level in EP infants (44.3±28.7 pg/mL) was significantly higher than that in LP infants (28.1±13.6 pg/mL) (P=0.006). There was not a significant difference in serum insulin level between EP and LP infants (372.7±254.2 pg/mL vs. 372.4±209.1 pg/mL, P=0.996). There was a significant difference in the serum glucagon level between infants with and without hypoglycemia (27.7±8.9 mg/dL vs. 36.8±24.6 mg/dL, P=0.036), but not in the serum insulin level (451.9±256.9 pg/mL vs. 357.4±222.2 pg/mL, P=0.211). Postnatal glucose concentration within the first 60 minutes of life had a significant positive correlation with serum glucagon levels (r=0.256, P=0.034), but not with serum insulin levels (r=–0.020, P=0.867).
Conclusion
Lower glucagon levels of cord blood were seen in premature infants with higher GA, which might contribute to the occurrence of postnatal hypoglycemia.
Introduction
Fetus is dependent on the mother for energy metabolism and the synthesis of other metabolic substrates1). At birth, the blood glucose concentration in the umbilical vein is 80%–90% of the prevailing maternal blood glucose concentration23), and the supply of glucose abruptly ceases. The glucose concentration falls rapidly, reaching a nadir by 1 hour of age and stabilizes by 3 hours of age even in the absence of exogenous glucose infusion34). Umbilical cord clamping leads to decrease in insulin levels and an acute surge in circulating counterregulatory hormones (glucagon, catecholamines, cortisol) levels25). These hormones stimulate hepatic glycogenolysis and gluconeogenesis to maintain the plasma glucose concentration56). In term neonates, exogenous glucose infusions such as enteral feeding achieve variable suppression of endogenous glucose production78). Therefore, the plasma glucose concentration in full-term healthy neonates is maintained at a steady 40–80 mg/dL and tolerates fasting. However, transient hypoglycemia is often a consequence of changes in the metabolic environment in utero or ex utero, influenced by factors, such as maternal diabetes, maternal drug treatment, intrauterine growth restriction, perinatal asphyxia, hypoxia, hypothermia, and infection910). However, insulin, glucagon, and human growth hormone levels did not differ from those of infants who remained normoglycemic1112).
Premature infants are at risk of hypoglycemia due to limited glycogen stores, lower gluconeogenic hormones activities, and decreased hormonal responses131415). Moderate neonatal hypoglycemia in preterm infants can lead to adverse neurodevelopmental outcomes1617). Bagnoli et al.18) reported that very low gestational age (GA) is characterized by higher glucagon plasma levels and insulin resistance, both of which may be determinants in the pathogenesis of neonatal hyperglycemia. In the literature, information about the insulin and glucagon levels of cord blood in preterm infants with hypoglycemia is lacking.
Therefore, here we aimed to determine whether serum insulin and glucagon levels of umbilical cord blood correlate with subsequent postnatal hypoglycemia in AGA - preterm infants at different GAs.
Materials and methods
This prospective study was conducted in the perinatal center of Keimyung University Dongsan Medical Center from October 2014 to March 2015. This study enrolled preterm infants born at 23–34 weeks' gestation. GA was estimated from the mother's last menstrual period. Exclusion criteria were being born to a diabetic mother, being small for GA (SGA), having a congenital anomaly, the presence of perinatal asphyxia and early onset sepsis. SGA was defined as a weight below the 10th percentile for GA according to Korean reference for birth weight (BW) by GA and sex19).
A total of 74 AGA infants, born at Keimyung University Dongsan Medical Center, Daegu, South Korea, were enrolled in this study. In all enrolled infants, initial resuscitation practices followed the most recent guideline, which was updated in 2010 by the International Liaison Committee on Resuscitation and the American Heart Association20). Perinatal asphyxia was defined as a 5-minute Apgar score ≤5. Three infants with perinatal asphyxia were excluded.
There were no infants whose mothers receiving intravenous dextrose during delivery. Infants were screened for capillary glucose levels by the reagent strips method within the first 60 minutes of life, before exogenous glucose infusion such as enteral feeding or intravenous dextrose injection. Postnatal hypoglycemia was defined as a capillary glucose level <40 mg/dL, regardless of GA. All infants who developed postnatal hypoglycemia were administrated an intravenous glucose infusion (bolus of 2 mL/kg 10% dextrose, and continuous glucose infusion at 4–7 mg/kg/min). Two infants with persistent hypoglycemia were excluded.
Sixty-nine infants were stratified according to GA: GA 23–30 weeks, early preterm (EP, n=31); GA 31–34 weeks, late preterm (LP, n=38).
The following potentially confounding variables were analyzed: GA, BW, ponderal index, delivery mode, body temperature at admission, antenatal steroid, premature rupture of membrane (PROM), respiratory distress syndrome (RDS), 1- and 5-minute Apgar scores. All infants with RDS were given an exogenous surfactant replacement via endotracheal intubation prior to the screening for capillary glucose levels.
1. Analytical procedures
Umbilical cord blood was collected from the vein of a double-clamped segment of the umbilical cord, into a Vacutainer rapid serum tube (BD Bioscience, San Jose, CA, USA). The serum of umbilical cord blood was immediately separated by cold centrifugation, divided into aliquots, and stored at –20℃ until processing. Insulin and glucagon levels of umbilical cord blood were measured by commercially available multiplex immunoassays (Luminex 200, Merck Millipore Corp., Darmstadt, Germany). The multiplex panels used in this study were Human Metabolic Hormone Magnetic Bead Panel (Cat. #HMHEMAG-34K-03) (Merck Millipore Corp., Darmstadt, Germany). The assays were performed according to the manufacturer's instructions. The capillary glucose concentrations were measured using the reagent strips method from Accu-Chek Performa (Roche Diagnostics, Indianapolis, IN, USA) within the first 60 minutes of life (range, 20–60 minutes).
2. Statistical analysis
Results are given as mean±standard deviation. Comparisons of demographic and clinical characteristics were performed using the chi-square test or Fisher exact test for categorical variables, and Student t-test for continuous variables. The Pearson correlation coefficient was used to analyze the data, and level of significance was set at α≤0.05 (P≤0.05). A P<0.05 was considered statistically significant. The IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the analyses.
3. Ethics statement
This study was approved by the Keimyung University Dongsan Medical Center Institutional Review Board (approval number: 2014-09-007). The need for informed consent was waived by the board because of the prospective study nature.
Results
Table 1 lists the clinical characteristics between EP and LP infants. The mean GA and BW in the EP group was significantly lower than that of the LP group (P=0.000). However, the ponderal index did not differ between the 2 groups (P=0.539). The mean body temperature at admission of the EP group was significantly higher than that of the LP group (36.1℃±0.3℃ vs. 35.8℃±0.4℃, P=0.000). Both 1- and 5-minute Apgar score in the EP group was significantly lower than those of the LP group (P<0.05). The use of antenatal steroid in the EP group was significantly higher than that in the LP group (P=0.016), but the incidence of PROM was not (P=0.271). The incidence of RDS in the EP group was significantly higher than that in the LP group (100.0% [31 of 31] vs. 50.0% [19 of 38], P=0.000). However, the mean capillary glucose concentration within the first 60 min of life in LP infants with RDS (56.4±20.6 mg/dL, n=19) was not significantly different from that in LP infants without RDS (55.4±13.7 mg/dL, n=19) (P=0.859). The incidence of postnatal hypoglycemia in EP infants (12.9%, 4 of 31) was lower than in LP infants (27.0%, 7 of 38), but the difference was not statistically significant (P=0.533).
Postnatal hypoglycemia developed in 11 infants (15.9%). Table 2 lists the differences between infants with and without postnatal hypoglycemia. The use of antenatal steroid, the incidence of RDS, mean body temperature at admission, 1-min and 5-minute Apgar scores did not differ significantly between the 2 groups (P>0.05).
The serum insulin and glucagon levels of cord blood between EP (n=31) and LP (n=38) infants are shown in Fig. 1. The serum glucagon levels of cord blood in EP infants (44.3±28.7 pg/mL) was higher than those in LP infants (28.1±13.6 pg/mL), and the difference was statistically significant (P=0.006). There was not a significant difference in the serum insulin levels of cord blood between EP and LP infants (372.7±254.2 pg/mL vs. 372.4±209.1 pg/mL, respectively, P=0.996). The capillary glucose concentration within the first 60 minutes of life in EP infants (65.5±21.2 mg/dL) was significantly higher than those of LP infants (55.9±17.3 mg/dL), and the difference was statistically significant (P=0.043).
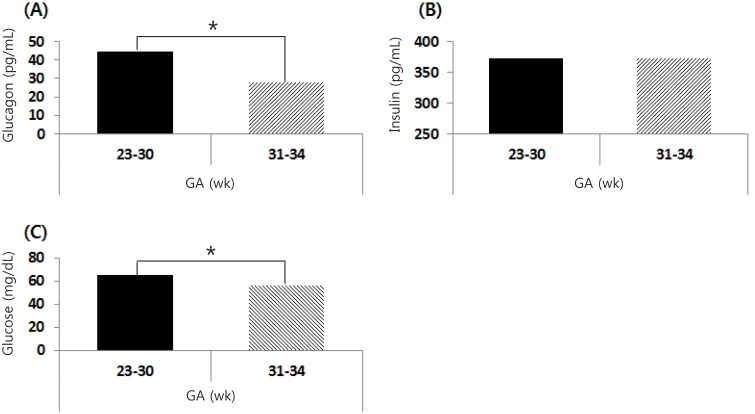
Serum glucagon (A) and insulin (B) levels in cord blood at birth. (C) The capillary glucose concentrations within the first 60 minutes of life. Glucagon and glucose index were significantly higher in early preterm (GA, 23–30 weeks) than in late preterm (GA, 31–34 weeks) infants. GA, gestational age. *P<0.05.
As shown in Fig. 2, there were significant differences in the serum glucagon levels of cord blood between infants with and without hypoglycemia (27.7±8.9 pg/mL vs. 36.8±24.6 mg/dL, respectively; P=0.036), but in the serum insulin levels of cord blood (451.9±256.9 pg/mL vs. 357.4±222.2 pg/mL, respectively; P=0.211).
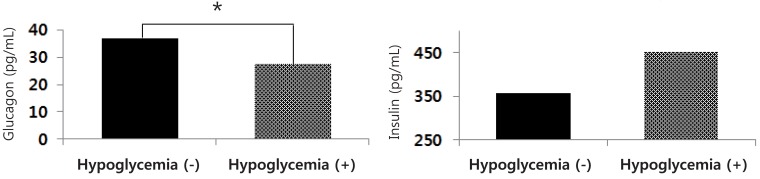
Serum glucagon and insulin levels of cord blood in preterm infants with and without postnatal hypoglycemia. GA, gestational age. *P<0.05.
An analysis of the linear correlation with capillary glucose concentration within the first 60 minutes of life is shown in Table 3. The capillary glucose concentration within the first 60 minutes of life in AGA-preterm infants was positively correlated with serum glucagon levels of cord blood (r=0.256, P=0.034), but not with serum insulin levels of cord blood (r=–0.020, P=0.867).
Discussion
The serum glucagon levels of umbilical cord blood in EP infants were significantly higher than those in LP infants. Glucose concentration within the first 60 minutes of life was significantly positively correlated with the serum glucagon levels of cord blood, but not with serum insulin levels. The mean glucose concentration within the first 60 minutes of life in EP infants was higher than that in LP infants. In conclusion, lower glucagon levels in the cord blood were seen in preterm infants with a GA, which might be a determinant of postnatal hypoglycemia.
After birth, transient neonatal hypoglycemia is related to conditions or events occurring during delivery. Perinatal asphyxia is a known risk factor of hyperinsulinism in the neonate owing to the anaerobic metabolism required to maintain blood glucose concentrations21). Infants born to mothers who received intravenous dextrose during delivery22) and were treated with hypoglycemic agents during pregnancy developed postnatal hypoglycemia23). SGA infants have a lack of glycogen and fat storage related to the risk for hypoglycemia24). According to previous studies, we excluded these infants from the current study.
Yoon et al.25) reported that the incidence of hypoglycemia (blood glucose concentration <40 mg/dL) during the first 2 hours in preterm infants (GA <34 weeks) was 12.8% and that blood glucose levels in the first 1 hour after birth was the highest in the GA<28 weeks group. In this present study, the mean glucose concentration within the first 60 minutes of life in the EP infants was higher than that in the LP infants.
Glucagon in the fetus stimulates glucose transfer across the placental barrier. The glucagon receptor is known to maintain maternal-fetal glucose transport as well as intrauterine growth26). The regulation of glucagon release is not obvious27). Bagnoli et al.18) reported that a very low GA was associated with high glucagon levels, which could be due to immaturity of the mechanisms controlling the hormone secretion. In the present study, EP infants with lower GA were associated with high glucagon levels, which could explain why the mean postnatal capillary glucose concentration in EP infants was higher than that in LP infants.
Lower GA and lower BW are the risk factor of hypothermia28). However, mean body temperature at admission in EP infants was significantly higher than that in LP infants in this present study. This significant difference in this study was associated with a different transport system from delivery room to neonatal intensive care unit as follows: EP infants transported by radiant warmer versus LP infants transported by a portable incubator without heating. Hypothermia is the risk factor of postnatal hypoglycemia in preterm infants. In the present study, body temperature at admission did not differ significantly between infants with and without postnatal hypoglycemia. Except for enviornmental factors, it could be necessary to investigate the correlation between body temperature and postnatal glucose concentration.
Before preterm delivery, the treatment of antenatal corticosteroids decreases the neonatal mortality and morbidites such as RDS, intaventricular hemorrhage, bronchopulmonary dysplasia, and necrotizing enterocolitis29). Koivisto et al.30) reported that the long exposure time to antenatal glucocorticoid was associated with the increased risk of postnatal hyperglycemia. In contrast to privious studies. On the contrary, Pettit et al.31) reported that antenatal steroid was associated with postnatal hypoglycemia, which was related to maternal hyperglycemia. In the present study, antenatal steroid administration did not differ significantly between infants with and without postnatal hypoglyemia. However, we did not investigate whether antenatal steroid induced maternal hyperglycemia. Further prospective studies are needed to evaluate the effect of antenatal steroid on postnatal glyemic control.
Padbury et al.32) reported that a surge of catecholamine from the fetal sympathoadrenal system was induced by postpartum stress, such as hypoxia. In this study, all EP infants underwent endotracheal intubation for the surfactant replacement of RDS. This could explain why the mean postnatal capillary glucose concentration in EP infants was higher than that in LP infants. However, further evaluations are needed to clarify whether the neonatal resuscitation affects postnatal glucose concentration.
This study was limited by factors such as a small number of infants and the lack of data on serum insulin and glucagon levels with hypoglycemic events.
In conclusion, lower glucagon levels of cord blood were seen in premature infants with higher GA, which might contribute to the occurrence of postnatal hypoglycemia.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2014R1A5A2010008).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.