Introduction
Cushing syndrome is caused by excessive circulating glucocorticoid concentrations caused by endogenous secretion or exogenous administration (iatrogenic Cushing syndrome)
1). Endogenous Cushing syndrome is subdivided according to adrenocorticotropic hormone (ACTH) dependency. ACTH-dependent Cushing syndrome is due to ACTH-secreting pituitary adenomas (Cushing disease), ectopic ACTH-secreting tumors, and ectopic corticotropin-releasing hormone (CRH) syndrome
1). A precise differential diagnosis of Cushing syndrome is often difficult due to the overlapping symptoms and signs among these subtypes. In particular, differential diagnosis between Cushing disease and ectopic ACTH-secreting tumors is difficult, even though there are helpful biochemical diagnostic tests or imaging studies such as magnetic resonance imaging (MRI) or scintigraphy
2). A pituitary MRI should be performed in all patients to diagnose ACTH-dependent Cushing disease, but this fails to identify the exact tumor origin and site in up to 50% of patients
3). Therefore, inferior petrosal sinus sampling (IPSS) is considered the gold standard for identifying both the origin of tumors and the site of a pituitary adenoma
4).
IPSS is a method that compares the blood level of ACTH collected from peripheral vessels and both sides of the inferior petrosal sinus after stimulation of ACTH secretion with CRH administration
5). Since 1995, desmopressin has been used in place of CRH due to the limited supply of CRH
6). Approximately 150 patients with Cushing disease tested with desmopressin have been reported on so far with the results showing sensitivity comparable to that of CRH stimulation
4). However, bilateral IPSS and use of desmopressin is uncommon in children, and there are few reports of the accuracy and complications of IPSS using desmopressin in children. We report the case of a 14-year-old girl successfully diagnosed with Cushing disease lateralized by IPSS with desmopressin.
Case report
A 14-year-old girl was referred to the Pediatric Endocrinology Clinic of Severance Children's Hospital for evaluation of the etiology of Cushing syndrome. In the 2 years prior to referral, the patient had experienced weight gain (20 kg/2 years), secondary amenorrhea, growth retardation, and back pain. Despite chiropractic adjustment for back pain, symptoms worsened and she was admitted to a tertiary hospital for conservative care and evaluation. During the diagnostic examination, spine MRI showed a compression fracture with osteoporosis, and further evaluations for osteoporosis and amenorrhea were performed. A random cortisol level (38.4 µg/dL) and 24-hour urinary free cortisol (UFC, 1280.0 µg/day; normal reference value, 10–34 µg/day) were elevated, suspecting Chshing's syndrome (
Table 1). Serum cortisol levels at 8 AM after an overnight dexamethasone suppression test (1 mg once) and low-dose dexamethasone suppression test (0.5 mg every 6 hours for 2 days) were not adequately suppressed (30.0 µg/dL and 11.9 µg/dL, respectively; normal reference value, <1.8 µg/dL), which confirmed Cushing syndrome (
Table 1). A midnight ACTH level was 32 pg/mL, which is greater than 22 pg/mL, confirming that the underlying disease was ACTH-dependent. A subsequent high-dose dexamethasone suppression test (2 mg every 6 hours for 2 days) to confirm Cushing disease was performed. Serum cortisol (8.3 µg/dL) and 24-hour UFC (251.0 µg/day) were suppressed by more than 50% compared to baseline, supporting a definite diagnosis of Cushing disease (
Table 1). However, pituitary MRI failed to identify a lesion and the patient was referred to our institute for further evaluation of the etiology of Cushing syndrome.
On admission, the patient's height and weight were 142 cm (<3rd percentile) and 53.5 kg (50th–75th percentile), and her BMI was 26.58 kg/m
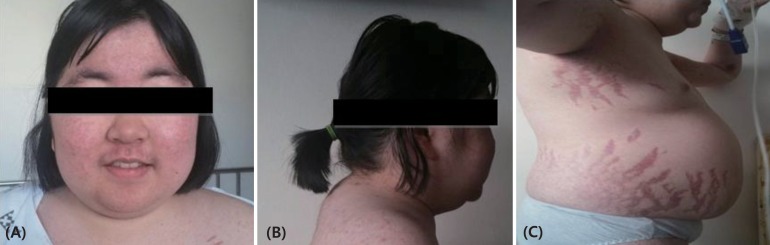
2. Hypertension (149/109 mmHg) was observed with a normal heart rate (96 beats/min), body temperature (36.9℃), and respiratory rate (16 breaths/min). She appeared chronically ill, with diffusely thin, wrinkled skin with plethora. She displayed a moon-shaped face (
Fig. 1A), a buffalo hump (
Fig. 1B), truncal obesity, acanthosis nigricans, and abdominal striae (
Fig. 1C).
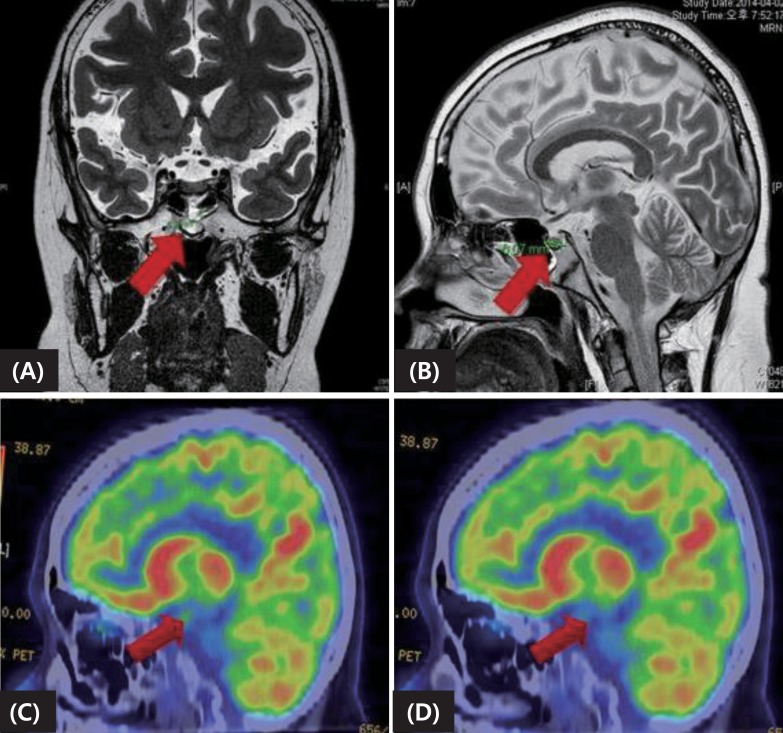
As her previous test results strongly suggested Cushing disease rather than an ectopic ACTH-producing tumor, a thin-slice pituitary MRI was performed. The MRI showed a heterogeneous enhancing lesion (approximately 6 mm) in the midline area of posterior aspect of the pituitary fossa, and pituitary microadenoma was suspected (
Fig. 2A, B). A positron emission tomography study also found diffuse 18-fluorodeoxyglucose uptake in an area consistent with the MRI which was not suppressed after dexamethasone, thus supporting the MRI findings of an adenoma (
Fig. 2C, D). An In-111 Octreoscan (Mallinckrodt Nuclear Medicine, Maryland Heights, MO, USA) showed no evidence of ectopic ACTH-secreting tumor.
Transsphenoidal surgery was considered the initial treatment of choice. As preservation of normal pituitary tissue is directly related to surgical success and prognosis
2), bilateral IPSS was performed by an expert neuroradiologist to determine the localization and laterality of ACTH secretion. The maximum ratio of ACTH concentrations between the inferior petrosal sinus and peripheral vein on the left side after injection of desmopressin was remarkably higher than that measured on the right side, suggesting left lateralization of ACTH secretion (
Table 2). Subsequently, total resection of the tumor by a transsphenoidal approach through the left nostril was performed. Yellow, slightly solid tissue was identified at the medial aspect of the left and lateral areas of the right cavernous space. Pathology of the biopsied tissue confirmed a pituitary adenoma. Postoperative MRI showed no gross residual tumor, and there was no significant complication. Serum ACTH and cortisol levels measured on the day after surgery were markedly decreased (<1.0 pg/mL and 1.8 µg/dL, respectively), which confirmed that the surgery had been successful (
Table 1). The patient was prescribed a long-term oral glucocorticoid 15 mg/body surface area, and was discharged. Four months after surgery, mild cushingoid features remained, but were much improved compared to the initial morphology. Laboratory data showed no evidence of recurrence (
Table 1). The patient is now tapering oral glucocorticoid (7 mg/body surface area), taking oral calcium and vitamin D, and receiving recombinant growth hormone therapy after confirming growth hormone deficiency on the combined pituitary function test. Long-term follow-up for linear growth, osteoporosis, adrenal insufficiency, and Cushing disease recurrence is required.
Discussion
Cushing syndrome comprises the symptoms and signs associated with prolonged exposure to inappropriately elevated levels of free plasma glucocorticoids from both endogenous and exogenous sources1). Classic features include centripetal obesity, moon face, hirsutism, and plethora, but systemic involvement is also seen
1). Obesity, hypogonadism, psychiatric abnormality, myopathy, hypertension, and infections are representative clinical manifestations of Cushing syndrome
1). In childhood, poor linear growth and weight gain are the most common manifestations, as in this case. Investigation of Cushing syndrome is done in 2 steps. The first is to confirm Cushing syndrome clinically and biochemically by checking the circadian rhythm of plasma cortisol and UFC excretion, and by performing an overnight or low-dose dexamethasone suppression test
1). The next step is to determine the cause, which includes checking levels of plasma ACTH, performing a high-dose dexamethasone suppression test, IPSS, MRI scanning of the pituitary and adrenal glands, scintigraphy, and testing for tumor markers
1).
IPSS is the most important test, especially in patients who do not present with a definite pituitary lesion. It was first performed in 1977 by Corrigan, and the protocol was later improved by Landolt through the addition of CRH stimulation
7). Because CRH is expensive and not widely available at present, desmopressin is emerging as an alternative to CRH
4). Vasopressin is one of the most powerful stimuli of ACTH secretion and desmopressin is a synthetic vasopressin analog. It binds to pituitary vasopressin type 3 (V3) receptors and stimulates ACTH secretion in a pituitary tumor that expresses V2 and V3 receptors
8). If the central/peripheral ACTH ratio determined by IPSS increases by more than two prior to administration of desmopressin, and by more than three after administration of desmopressin, then Cushing disease due to microadenoma is strongly suggested. Studies of the efficacy of desmopressin have mostly yielded positive results. Deipolyi et al.
6) performed 20 cases of IPSS using desmopressin, and demonstrated 94.5% sensitivity, comparable with CRH stimulation
6). Among 36 cases in the literature, IPSS using desmopressin had a sensitivity of 95% for Cushing disease, and a specificity of 100% for ectopic ACTH secretion
7). Current case administered demopressin as a stimulant for IPSS, considering the wide availability, sensitivity and specifity of desmopressin compared with CRH.
However, lateralization by IPSS is controversial. Castinetti et al.
7) found low sensitivity (50%) in determination of the side of the adenoma in 36 cases of IPSS
7). In the current case, the peak central/peripheral ratio during right IPSS remained at 0.9–1, whereas the ratio on the left increased to a maximum of 20, suggesting left lateralization. The operative findings, which showed a tumor on the left side, were concordant with these results. Therefore, IPSS was thought to be the most reliable test to distinguish between Cushing disease and ectopic ACTH syndrome, particularly in cases where current imaging techniques have limitations. Additionally, IPSS can provide more precise information about the localization of the tumor to the neurosurgeon for successful resection
4).
In our case, there were no serious complications during IPSS, such as venous thrombosis, pulmonary embolism, cranial nerve palsy, or brainstem vascular damage
5). However, desmopressin use mandates additional precautions compared to CRH, as this agent has a known hemostatic action and promotes the release of von Willebrand factor
9). As IPSS is an invasive technique, further use of desmopressin might require additional precautions by specialists who perform the procedure.
In conclusion, IPSS using desmopressin has rarely been reported in children. Our case suggests that desmopressin use instead of CRH to stimulate ACTH in pediatric patients may be safe and effective. IPSS can be a valuable tool not only for discriminating between pituitary origin and ectopic ACTH-secreting Cushing syndrome, but also for providing lateralization of pituitary adenomas.