The impact of obesity on hyperandrogenemia in Korean girls
Article information
Abstract
Purpose
As metabolic complication and polycystic ovarian syndrome due to childhood obesity is rising, the role of hyperandrogenemia (HA) and hyperinsulinism is receiving attention. The aims of this study were to investigate the presence of obvious HA according to pubertal status and to find potential etiologic determinants of HA in Korean obese (OB) girls.
Methods
We analyzed 91 girls aged 6–17 years (prepuberty, n=54; puberty, n=37). Each girl was classified as being either normal weight (NW) or OB. Anthropometric measurements were obtained and blood test was performed early in the morning after at least 8 hours of fasting to measure glucose, insulin, total testosterone, sex hormone-binding globulin, dehydroepiandrosterone sulfate (DHEAS), luteinizing hormone (LH), follicular-stimulating hormone, estradiol, and progesterone.
Results
The plasma levels of free testosterone (FT) and DHEAS were markedly higher in OB girls compared to NW girls in puberty (FT, P=0.009; DHEAS, P=0.046) but not in prepuberty (FT, P=0.183; DHEAS, P=0.052). Hyperinsulinemia and high homeostasis model assessment of insulin resistance (HOMA-IR) values were found regardless of pubertal status in OB girls. The significant related factor to HA in puberty was the body mass index Z-score (P=0.003). But HOMA-IR, LH, and progesterone levels were not relevant to HA in pubertal girls.
Conclusion
OB prepubertal girls did not show HA in the present study but they should be regularly monitored because they already had hyperinsulinemia. OB pubertal girls had significant HA and hyperinsulinemia, and obesity per se was the most important factor for HA.
Introduction
Childhood obesity has increased all over the world in the past decades and Korea is no exception1). As the impact of childhood obesity on adulthood metabolic complications is considered important2), the polycystic ovarian syndrome (PCOS), which is a reproductive complication3), is also receiving attention. PCOS, a disorder of ovarian dysfunction, is characterized by relatively high androgen concentrations, metabolic abnormalities and many other conditions of the metabolic syndrome4). Because PCOS patients may enter the hyperandrogenism-hyperinsulinism cycle, there is no doubt that obesity plays a significant role5). There are many efforts to reveal the definite interacting mechanisms between obesity, hyperinsulinemia, hyperandrogenemia (HA), and gonadotropins467). Hyperandrogenism, which was selected as one of the critical criteria for PCOS by Androgen Excess Society in 20068), was reported as a significant risk factor for metabolic syndrome in PCOS patients9).
Clinical manifestations of PCOS may develop even in adolescence3), often begin during or soon after puberty. And HA during early adolescence may represent a precursor of adult PCOS10), thus, early pubertal obesity and HA should not be overlooked. Recent insights suggest that peripubertal obesity was associated with marked HA, which was pronounced in prepuberty and early puberty1112). However, other studies reported that not all obese (OB) girls have HA and some of early pubertal OB girls had normal androgen concentrations1314). Therefore, the presence of HA and its role in childhood and adolescence is not clear to date.
There have been proposed various preceding factors of HA. Increased fat compartment is a well-known factor15). Weight loss induced a decrease in testosterone levels in OB prepubertal and pubertal girls, pointing to HA as a reversible phenomenon16). But obesity per se is not sufficient to produce HA14). Luteinizing hormone (LH), which is a major physiological stimulus for ovarian androgen production from theca cells, is related to HA6). And hyperinsulinemia contributes to HA through several mechanisms171819) including potentiation of LH action.
To our knowledge, this is the first study of HA in OB Korea girls including prepubertal age. The prevalence of morbid obesity is relatively low in Korea compared to the Western Countries20) and metabolic consequences according to the same body mass index (BMI) are different among ethnic groups. Therefore, we aimed to investigate the presence of obvious HA and to find potential etiologic determinants of HA in both prepubertal and pubertal Korean OB girls
Materials and methods
1. Subjects
Between June 2007 and May 2008, 99 girls aged 6–17 years who visited the Hallym Medical Center outpatient clinics due to their health check-ups were enrolled retrospectively. All participants and their ancestors were Korean. Anthropometric measurements were obtained from each subject. Height was measured twice to the first decimal place with a Harpenden stadiometer (Holtain Ltd., Crosswell, UK) and weight to the first decimal place with a digital scale (150A, Cas Co. Ltd., Seoul, Korea). To calculate BMI, weight was divided by the square of the height (kg/m2). With the measured height and weight, BMI Z-scores were obtained by using Korean growth standard published by the Korea Centers for Disease Control and Prevention21). Each girl was classified as being either normal weight (NW) (BMI adjusted for age between 5th–84th percentile) or OB (BMI adjusted for age≥95th percentile). Waist and hip circumferences were measured at a horizontal level 1 inch above the navel and around the widest portion of the buttocks, respectively. Waist to hip ratio (WHR) was also calculated. Tanner stage was evaluated by a single female pediatric endocrinologist who conducted both inspection and palpation of breasts. Subjects were assigned to prepuberty (Tanner breast stage I) or puberty (Tanner breast stage IV–V). Exclusion criteria were as follows: underweight, BMI adjusted for age below 5th percentile; overweight, BMI adjusted for age between 85th–94th percentile; Tanner breast stage II–III. All subjects had a blood test early in the morning after at least 8 hours of fasting. All subjects had taken no medications known to affect the reproductive axis for at least 3 months before the study. This study protocol was approved by the Institutional Review Board of Hallym Medical Center (KANGDONG 2016-08-002).
After study enrollment, 8 subjects were excluded as follows: one subject whose fasting plasma glucose level was 295 mg/dL; and 7 subjects with a morning progesterone concentration greater than 2 ng/mL in Tanner stage IV–V, which sampling is during the luteal phase14). Therefore, 91 subjects were included in this study and the number of subjects in NW prepuberty, OB prepuberty, NW puberty, and OB puberty group was 24, 30, 11, and 26, respectively. Because there was 12 missing data in waist or hip circumferences, WHR was calculated in 79 subjects.
2. Hormone assays
Glucose was measured by enzymatic reference method with hexokinase (Roche Diagnostics, Indianapolis, IN, USA): sensitivity, 2 mg/dL. Insulin, progesterone, and sex hormone-binding globulin (SHBG) were measured by electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN, USA). LH and follicular-stimulating hormone (FSH) were measured by IRMA (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Total testosterone (TT), dehydroepiandrosterone sulfate (DHEAS), and estradiol were measured by RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Sensitivity, interassay and intra-assay coefficients of variation for each hormone were as follows: insulin, 0.1 µU/mL, 2.5%–2.8%, and 1.9%–2.0%; progesterone, 0.03 ng/mL, 4.1%–5.5%, and 1.5%–2.7%; SHBG, 0.35 nmol/L, 1.8%–4.0%, and 2.7%–5.6%; LH, 0.1 IU/L, 3.2–4.1, and 5.3%–6.6%; FSH, 0.05 IU/L, 2.2%–2.3%, and 5.9%–6.3%; TT, 0.1 ng/mL, 7.8%–9.3%, and 1.5%–6.1%; DHEAS, 0.1 µg/dL, 3.4%–10.6%, and 3.5%–7.4%; and estradiol, 0.1 pg/mL, 4.2%–8.1%, and 4.0%–7.0%.
Free testosterone (FT) was calculated from TT and SHBG using the following equation22): FT=[TT–(N)(FT)]/[(KT) (SHBG) – (KT)(TT) + (N)(KT)(FT)]. In this equation, FT is free testosterone concentration (pmol/L); TT is total testosterone concentration (nmol/L); SHBG is SHBG concentration (nmol/L); KT is the association constant of SHBG for testosterone (1.0×109 L/mol); N=(KA)(CA)+1, where KA is the association constant of albumin for testosterone (3.6×104 L/mol), and CA is the albumin concentration (≒4.3 g/dL). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting plasma glucose and insulin using the equation as follows: HOMA-IR=Glucose (mg/dL)×insulin (µIU/mL)/40523).
3. Statistics
All data were presented as median and interquartile ranges. Mann-Whitney U-test or chi-square test was used to examine differences between the 2 groups, classified according to BMI and Tanner stage. Spearman correlation coefficient was calculated to test the association between 2 continuous parameters. All hormone variables were highly skewed and logarithmic transformation was performed before multivariate regression analysis which was used to determine factors related to HA after controlling for other variables. P-values <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
Results
1. Clinical characteristics of subjects and insulin related indices
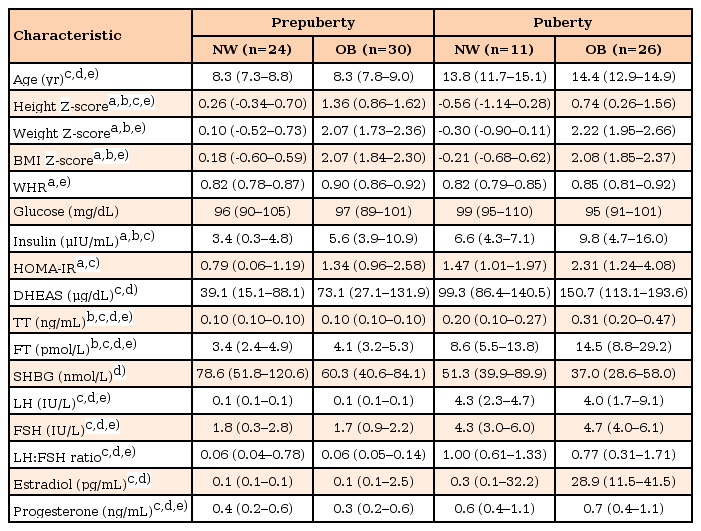
Clinical characteristics and hormone results of each group are presented in Table 1. Height Z-score of OB subjects was higher than that of NW subjects in both prepuberty and puberty. Birth weight of OB subjects was heavier than that of NW subjects in both prepuberty and puberty but all data was within normal range (median birth weight; NW prepuberty, 3.2 kg; OB prepuberty 3.4 kg; NW puberty, 2.5 kg; OB puberty, 3.3 kg). In total subjects, WHR showed positive correlation with BMI Z-score (r=0.421, P<0.001, n=79), fasting insulin level (r=0.244, P=0.030, n=79), and HOMA.IR (r=0.232, P=0.040, n=79). In prepuberty group, WHR showed good correlation with BMI Z-score (r=0.501, P<0.001, n=45), fasting insulin level (r=0.456, P=0.002, n=45), and HOMA.IR (r=0.438, P=0.003, n=45) but when considering for age, BMI Z-score, and HOMA-IR or insulin level all together, only BMI Z-score showed significance (P=0.006). In puberty group, WHR and BMI Z-score showed positive correlation with marginal significance (r=0.322, P=0.063, n=34) and it was not valid after controlling for age, and HOMA-IR or insulin.
Plasma glucose level was not different among the 4 groups. In all 91 subjects, HOMA-IR was correlated with age and BMI Z-score (adjusted R2=0.313; age, β=0.039, P=0.030; BMI Z-score, age, β=0.252, P<0.001). OB subjects had higher HOMA-IR values than NW subjects in prepuberty (P=0.002). But in puberty those differences were marginally significant (P=0.063). Plasma insulin level and HOMA-IR showed no differences between OB prepuberty group and NW puberty group (insulin, P=0.825; HOMA-IR, P=0.977) (Table 1, Fig. 1).
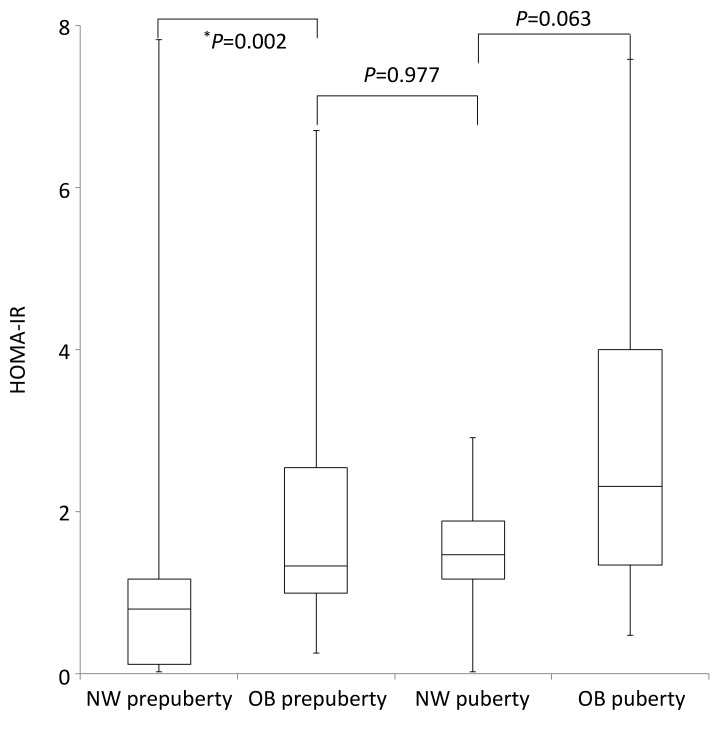
Box plots of homeostasis model assessment of insulin resistance (HOMA-IR) values of each group are shown. Obese (OB) subjects had higher HOMA-IR values than normal weight (NW) subjects in prepuberty (P=0.002). But in puberty those differences were marginally significant (P=0.063). HOMA-IR values of OB prepuberty group showed similar levels compared to those of NW puberty group (P=0.977). *P<0.05.
2. Androgen and gonadotropic hormone profiles
In puberty, plasma FT levels of OB subjects were about 2 times higher than those of NW subjects (P=0.018) (Fig. 2), while those were similar between OB and NW subjects in prepuberty (P=0.127). Plasma levels of TT and FT were higher in NW puberty group than in OB prepuberty group (both P<0.001). When age, log HOMA-IR, log LH and log progesterone were considered together in the multiple regression analysis, BMI Z-score and log progesterone showed significant correlation with the increased log FT in pubertal girls (Table 2). In prepuberty, TT and FT levels were randomly distributed regardless of age, BMI Z-score, and other variables. SHBG showed negative correlation with BMI Z-score (r=–0.330, P=0.001) but there were no differences between OB and NW subjects in both prepuberty and puberty (Table 1).

Box plots of free testosterone levels of each group are shown. In prepuberty, free testosterone levels were similar between obese (OB) and normal weight (NW) groups (P=0.127), while in puberty, it was about 2 times higher in OB groups than in NW groups (P=0.018). *P<0.05.
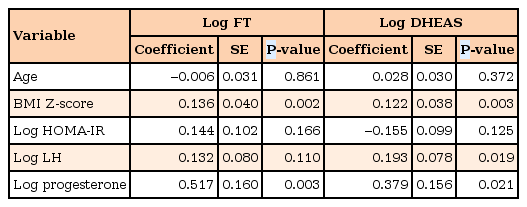
Multiple regression model of plasma levels of free testosterone and dehydroepiandrosterone sulfate in puberty subjects
In puberty, DHEAS levels showed patterns similar to FT levels but their differences between OB and NW subjects were marginally significant (P=0.060). In the multiple regression analysis, DHEAS levels of pubertal girls had significant correlations with BMI Z-score and plasma levels of LH and progesterone (Table 2). In prepuberty, DHEAS levels were not different between OB and NW subjects (P=0.180). There were no differences between OB prepuberty group and NW puberty group (P=0.131).
In total 91 subjects, plasma levels of LH, FSH, LH to FSH ratio, and progesterone were not associated with the BMI Z-score, while their differences were only affected by pubertal status. Within puberty subjects, plasma LH level was not correlated with BMI Z-score (r=–0.089, P=0.599, n=37) or HOMA-IR values (r=0.053, P=0.755, n=37) in the simple correlation analysis.
Discussion
In our study, hyperinsulinemia and high HOMA-IR values in OB subjects were confirmed regardless of pubertal status although they showed marginal significance in pubertal girls. But marked HA was found in OB girls compared to NW girls only after puberty. And significant factors related to HA was the BMI Z-score and progesterone levels, while, HOMA-IR and LH levels were not relevant to HA. In prepuberty, against our expectation, there was no evidence of HA in OB subjects despite they had higher HOMA-IR and insulin levels than NW subjects.
Recent data have disclosed a high prevalence of HA among peripubertal adolescents with obesity, suggesting that such girls are indeed at risk for developing PCOS3). The association between obesity and insulin resistance/hyperinsulinemia in childhood is already known24) and even in prepuberty, obesity with hyperinsulinemia is a cardinal matter which was also confirmed in our study. The reported mechanism of development of PCOS from obesity is via insulin resistance and compensatory hyperinsulinemia, which augments androgen production and suppresses SHBG, thereby increasing androgen bioavailability3). And obesity itself increases androgen production25) and peripheral conversion of androstenedione to testosterone in adipose tissue26). However, HA of OB peripubertal girls have conflicting results even in similar populations. McCartney et al.12) reported that 8.8 fold and 2.2 fold higher mean FT level in OB than in non-OB Tanner I–II girls, respectively. While they could not find significant differences in overnight FT levels between OB and non-OB Tanner I–II girls in another study13). It could be interpreted as that not only obesity or hyperinsulinemia but also other factors such as pubertal levels of gonadotropins are essentially included in the pathogenesis of HA, as it is indicated through other studies6). Insulin is able to decrease the hepatic production and secretion of SHBG27). Moreover, insulin amplifies the stimulatory effect of LH on ovarian theca cells to increase androgen production1719), can increase adrenal responsiveness to adrenocorticotropin hormone for further androgen production18), and can cause pituitary hyperresponsiveness to gonadotropin-releasing hormone for increased LH secretion28). The findings of this study, HA only in OB puberty not in OB prepuberty compared to NW subjects respectively, might be in the same line with the importance of the role of LH. FSH and insulin like growth factor-1 (IGF-1) also have been postulated to influence ovarian androgen production14). Therefore, multifactorial mechanisms may be alleviate the impact of hyperinsulinemia or HOMR-IR on HA in our study.
As log LH was related to log DHEA in puberty subjects in our study, some studies reported that higher morning LH concentrations independently predict increased FT level better than fasting insulin, BMI Z-score, age, pubertal stage, and IGF-114). However, Rosenfield and Bordini29) mentioned that LH elevation appears to be secondary to HA and is absent in the most OB cases, so it does not seem to cause the hyperandrogenism although it may aggravate it. The pattern of LH secretion, amplitude and frequency, is variable according to Tanner stage, daytime or nighttime, and fatness613). After puberty, LH levels show invert U-shape relative to BMI, though they are necessary for steroidogenesis29); therefore, mechanism of HA especially related to LH secretion is not yet confirmed. In addition, although the association of obesity with early sexual maturation is well described30), early signs of puberty (thelarche) do not generally reflect normal maturation of the hypothalamic-pituitary-ovarian axis in OB girls31). Thus, subjects with relatively immature gonadotropin-sex hormone axis might be included in OB pubertal group of this study. Although, LH:FSH ratios were not elevated in OB puberty group compared to NW puberty group in our study, women with PCOS have abnormally rapid LH pulses with reduced response to progesterone feedback, contributing to elevations in serum LH:FSH ratios32). Relative increase in LH leads to increased ovarian androgen synthesis and the resultant HA contributes to maintaining increased LH pulse frequency6). Thus when considering this vicious loop, the relationship between elevated progesterone levels and log FT or DHEAS in our study is understandable but whether progesterone level is the etiologic factor to HA is not sure.
Pubertal insulin resistance was confirmed in NW subjects. But plasma insulin levels or HOMA-IR values of OB prepuberty group were not lower than those of NW puberty group or OB puberty group. This was consistent with the other study which indicated that BMI was the predictor of insulin sensitivity changes during puberty33). Interestingly, DHEAS levels were similar between OB prepuberty group and NW puberty group in our study. It may because hyperinsulinemia stimulates abnormal adrenal steroidogenesis34), affecting DHEAS levels more directly than peripheral androgens. WHR is usually used as a visceral fatness index; WHR value above 0.86 is considered as having abdominal obesity, which is related to worse metabolic outcome than gluteal-femoral obesity (WHR<0.80)35). However, the present study showed that WHR was not correlated to insulin or HOMA-IR, when age and BMI Z-score were controlled. Therefore, in a clinical setting, measuring waist and hip circumferences might not be beneficial when considering time and the effort.
There are some limitations in our study. First, only one timed sample was collected. All samples were collected in the early morning but secretory dynamics of gonadotropins and sex hormones is not clear and nocturnal change of LH secretions should be considered together13). Second, bone age was not estimated. Bone age is advanced when a subject is OB through multiple mechanisms. For example, as reviewed above, insulin from adipose tissue related to obesity act on IGF-1 receptor. Therefore, height Z-score differences between NW and OB groups might be affected by bone age advancement especially in puberty. Third, although we excluded Tanner stage IV–V subjects with progesterone levels above 2 ng/mL, follicular or luteal phase should be identified for cases with mature menstrual cycle. Lastly, sample size of each group was small which could influence the level of statistical significance. In spite of these weaknesses, as Ibáñez et al.7) suggested, early recognition of girls at risk of developing hyperinsulinemic androgen excess is important for its prevention in childhood, and our study is worth as it included prepubertal OB girls even though they had not HA yet.
In conclusion, obesity per se was the most important factor for HA in OB pubertal girls who had significant HA and hyperinsulinemia. OB prepubertal girls did not show HA in the present study but they should be regularly monitored because they already had hyperinsulinemia.
Acknowledgments
This study was supported by the Pediatric Research Fund of Korean Society of Pediatric Endocrinology (grant No. 2007-03).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.