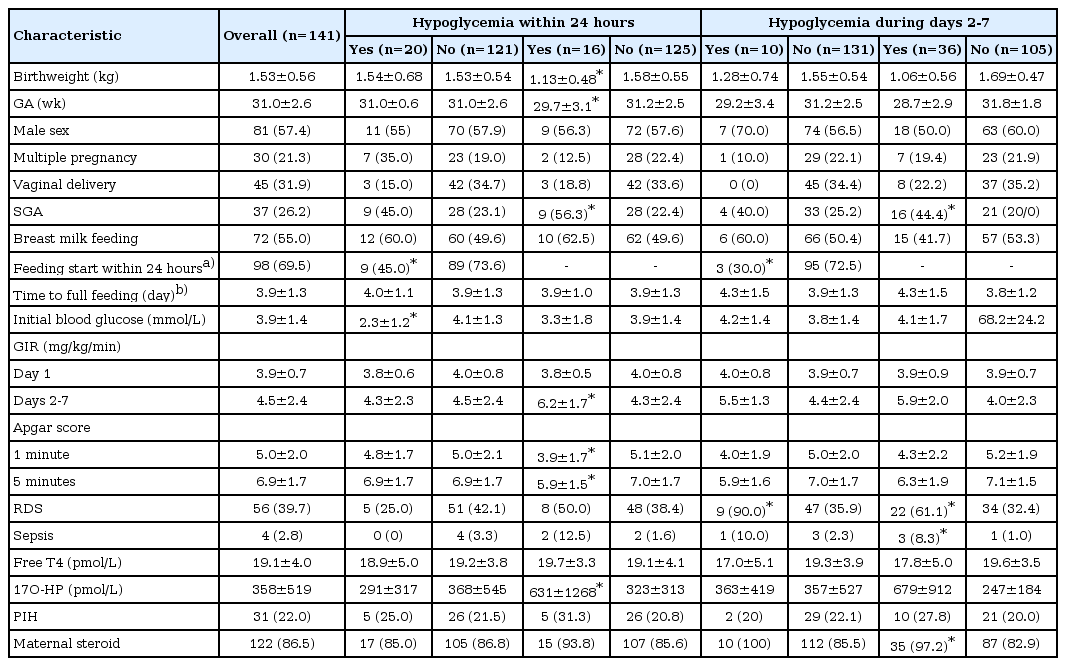
Blood glucose levels within 7 days after birth in preterm infants according to gestational age
Article information
Abstract
Purpose
This study investigated blood glucose levels in preterm babies according to gestational age (GA).
Methods
Subjects were 141 preterm infants with a GA<34 weeks. Data on blood glucose levels, GA, body weight, glucose infusion rate, and other contributing factors in the first 7 days after birth were analyzed. Hypoglycemia was defined as a blood glucose level of <40 mg/dL up to 24 hours after birth and as <50 mg/dL thereafter. Hyperglycemia was defined as a blood glucose level >180 mg/dL.
Results
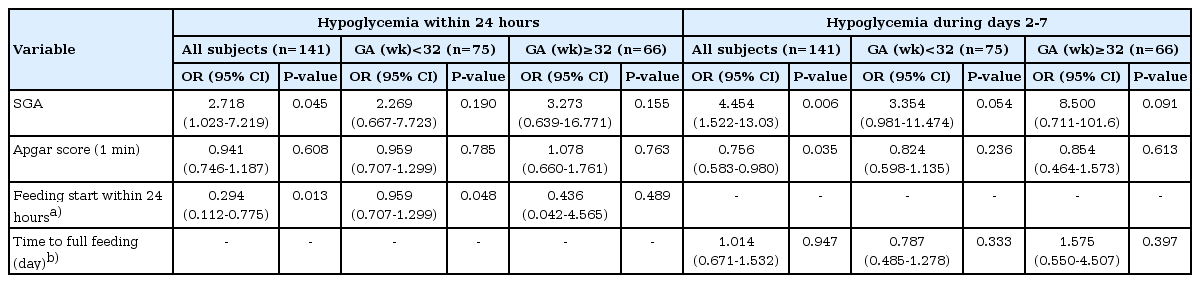
During the 7 days after birth, hypo- and hyperglycemia occurred in 29 (29 of 141, 20.6%) and 42 (42 of 141, 29.8%) neonates, respectively. During the first 2 hours, 18 neonates (12.8%) exhibited hypoglycemia, and only 2 (2 of 141, 1.4%) developed hyperglycemia. From 6 to 24 hours, hypo- and hyperglycemia were observed in 0 and 9 (9 of 141, 6.4%) neonates, respectively. Infants small for their GA (SGA) were at risk for hypoglycemia both within 24 hours (odds ratio [OR], 2.718; P=0.045) and during days 2 to 7 (OR, 4.454; P=0.006), and hyperglycemia during days 2 to 7 (OR, 3.200; P=0.005). Low 1-minite Apgar score was risk factor for both hypo- and hyperglycemia during days 2 to 7 (OR, 0.756; P=0.035 for hypoglycemia and OR, 0.789; P=0.016 for hyperglycemia). Both hypo- and hyperglycemia within 24 hours were less common in those who started feeding (OR, 0.294; P=0.013 for hypoglycemia and OR, 0.162; P=0.011 for hyperglycemia).
Conclusion
Careful blood glucose level monitoring is required in preterm infants, especially SGA infants or those with low Apgar score. Early feeding could be beneficial for maintaining euglycemia.
Introduction
Preterm infants have limited supplies and stores of energy sources for carbohydrate metabolism. In addition, the organs involved in the regulation of energy metabolism, which include the liver, pancreas, brain, and endocrine organs, are immature1). Therefore, hypo- and hyperglycemia are more common in preterm infants than in full-term neonates. Hypoglycemia is also common in infants who are small for gestational age (SGA)2), as well as in those with perinatal asphyxia3). Hyperglycemia occurs more frequently under conditions of excess glucose and lipid infusion, as well as under stressful conditions such as mechanical ventilation and hypoxia.
Persistent and recurrent hypoglycemia in neonates is associated with long-term neurological complications such as visual defects4), localization-related epilepsy567), and cognitive dysfunction8910). Complications of hyperglycemia include intraventricular hemorrhage, premature retinopathy, and bronchopulmonary dysplasia11).
However, little is known about prevalence of hypo- or hyperglycemia in preterm infants, resulting in challenges for prevention and management. The present study aimed to provide useful data for the prevention and treatment of hypo- or hyperglycemia in preterm infants by investigating blood glucose levels for a period of 7 days after birth, as well as the prevalence of and risk factors for these conditions.
Materials and methods
1. Subjects
Preterm infants born at a gestational age (GA) of <34 weeks at Seoul National University Bundang Hospital, Korea, were enrolled in this study. Of the 156 preterm infants born at a GA of <34 weeks between March 2010 and December 2011, 15 were excluded from the study (due to death within 7 days of birth [n=8] or maternal diabetes mellitus [n=7]). Finally, a total of 141 infants were enrolled in this study.
2. Methods
Blood glucose levels were measured irrespective of feeding time at least twice a day for the first 3 days after birth and at least once a day from days 4 to 7. Whole blood samples were obtained from the heel using the OneTouch SureStep Hospital Meter (LifeScan Inc., Milpitas, CA, USA). Because there is no consensus about definition of hypo and hyperglycemia, we used our target blood glucose level for cutoff value. Hypoglycemia was defined as a blood glucose measurement <2.22 mmol/L (40 mg/dL) within 24 hours of birth and <2.78 mmol/L (50 mg/dL) thereafter. Hyperglycemia was defined as a blood glucose measurement >9.99 mmol/L (180 mg/dL) regardless of the sampling time.
Patients were infused with a glucose-containing fluid at an initial glucose infusion rate (GIR) of 4 mg/kg/min. The GIR was titrated by 0.5 to 1 mg/kg/min daily up to 12 to 15 mg/kg/min, to maintain blood glucose levels within target range of 2.78-9.99 mmol/L (50-180 mg/dL). Hypo- and hyperglycemia were managed by adjusting glucose infusion rate. Feeding was initiated on the first day, if tolerable.
In addition to the measurement of blood glucose levels, sex, GA, birth weight, multiple pregnancies, method of delivery, prenatal steroid use, and maternal hypertension were recorded. We further collected information on perinatal factors associated with blood glucose levels, including GIR, feeding (breast milk or whole milk), and Apgar scores. In order to evaluate the hormonal status, free thyroxine (T4) and 17-hydroxyprogesterone (17-OHP) levels were measured on day 7 by serum radioimmunoassay and the neonatal screening test, respectively.
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No.: B-1203/148-101).
3. Statistical analysis
Results are expressed as the mean±standard deviation. Intergroup comparisons were conducted using independent t-tests or the chi-square test where appropriate. In addition, stepwise logistic regression analysis was performed to determine those factors influencing the occurrence of hypo- or hyperglycemia. These results are expressed as the odds ratios (ORs) with 95% confidence intervals (CIs).
Analyses were performed using the IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). A P-value <0.05 was considered statistically significant.
Results
1. Patient characteristics
The mean birth weight of all subjects was 1.53±0.56 kg. Of these, 37 infants (26.2%) were classified as SGA.
The subjects were divided into 4 groups according to GA: <28, 28 to <30, 30 to <32, and ≥32 weeks. No differences were observed between groups in terms of characteristics such as sex, multiple births, and method of delivery (vaginal vs. Cesarean section).
With regards to those perinatal factors that could affect blood glucose levels, as the group was younger, Apgar scores at 1 and 5 minutes were significantly lower, free T4 levels were lower, 17-OHP levels were higher, and a greater proportion of subjects developed respiratory distress syndrome. Feeding method, GIR during day 1, and maternal factors such as maternal hypertension or steroid use were not significantly different between the 4 groups (Table 1).
We grouped the subjects according to presence of hypo- and hyperglycemia. Infants who developed hypoglycemia within 24 hours had lower Apgar score than who did not. Those who developed hypoglycemia during days 2 to 7 had lower birthweight and Apgar score, and were younger than those who did not. Group with hyperglycemia during days 2 to 7 had higher proportion of SGA, and also reported higher prevalence of respiratory distress syndrome and sepsis (Table 2).
2. Blood glucose levels
An average of 17.5 samples per infant were done within 7 days of birth. The median blood glucose level 1 hour after birth was 3.66 mmol/L (66 mg/dL), the lowest level recorded throughout the study period, whereas the highest measurement was 6.38 mmol/L (115 mg/dL), obtained on day 7. The 2.5th-percentile level was lowest at 1.05 mmol/L (19 mg/dL) at 2 hours, and peaked at 3.4 mmol/L (62 mg/dL) on day 6. The 97.5th-percentile blood glucose level was 6.71 mmol/L (120.7 mg/dL) at 1 hour and peaked at 15.4 mmol/L (276.5 mg/dL) at day 7 (Figs. 12). The 5th blood glucose levels were lowest at 2 hours after birth at 1.89 mmol/L (34 mg/dL) (data not shown).
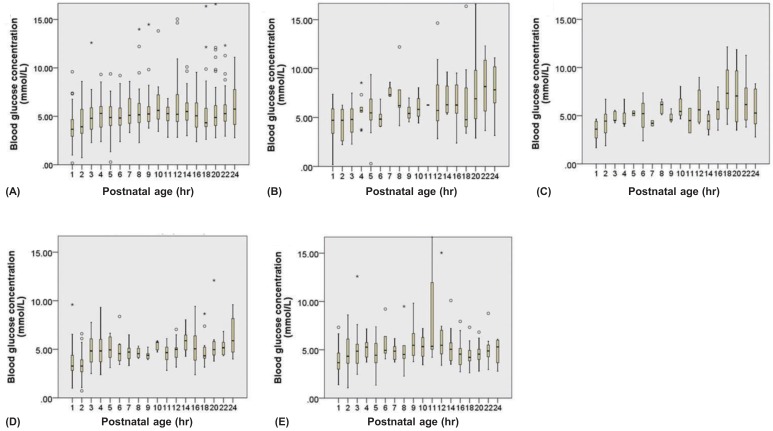
Blood glucose levels within 24 hours. (A) In all the subjects; (B) subjects with gestational age<28 weeks; (C) 28 to <30 weeks; (D) 30 to <32 weeks; and (E) ≥32 weeks. Lower and upper limit are mean±2standard deviation. Bars express inter quartile range. *Outlier.

Blood glucose levels over 7 days. (A) In all the subjects; (B) subjects with gestational age <28 weeks; (C) 28 to <30 weeks; (D) 30 to <32 weeks; and (E) ≥32 weeks. Lower and upper limit are mean±2standard deviation. Bars express inter quartile range. *Outlier.
During the first 2 hours, 18 neonates (12.8%) exhibited hypoglycemia, while hyperglycemia developed in just 2 neonates (1.4%). From 6 to 24 hours, hypo- and hyperglycemia were observed in 0 (0%) and 9 neonates (6.4%), respectively. Overall, 29 (20.6%) and 42 neonates (29.8%) developed hypoand hyperglycemia over 7 days, respectively. Eleven infants (7.8%) developed both hypo- and hyperglycemia, whereas 81 (57.4%) showed neither.
3. Blood glucose levels according to GA
At 1 hour after birth, median blood glucose levels were lowest in infants with a GA of 30 to <32 weeks and highest in those with a GA <28 weeks (3.27 and 4.72 mmol/L, respectively) (Fig. 1). Median blood glucose levels were lower on the first day in all GA groups. Median blood glucose levels peaked at day 7 in infants with a GA <32 weeks and showed steady levels, ranging from 3.67 to 5.44 mmol/L (66 to 98 mg/dL), in those with a GA≥32 weeks (Fig. 2).
4. Hypo-/hyperglycemia according to GA
During the first 24 hours, hypoglycemic episodes were most common in the GA 30 to <32 weeks group (20%, 3 of 15 patients) and least common in those with a GA<28 weeks (9.5%, 2 of 21 patients), although these intergroup differences were not statistically significant. Hyperglycemia was higher in the younger GA group (19%, 6.7%, 5.1%, and 4.5% in GA< 28, 28 to <30, 30 to <32, and ≥32 weeks, respectively), although not statistically significant (Table 1).
During days 2 to 7, hyperglycemia occurred more frequently in the younger age groups; 16 of 21 neonates (76.2%) with a GA<28 weeks developed hyperglycemia, while only 6 of 66 neonates (9.1%) with a GA≥32 weeks exhibited hyperglycemia. No significant differences regarding the incidence of hypoglycemia were observed among the groups (Table 1).
5. Risk factors for hypoglycemia
Univariate logistic regression analysis was performed to determine risk factors for hypoglycemia. The results showed that SGA was significant risk factors for hypoglycemia within 24 hours (OR, 2.718; 95% CI, 1.023-7.219). Having started feeding was related with decreased hypoglycemia within 24 hours (OR, 0.294; 95% CI, 0.112-0.775). For hypoglycemia during days 2 to 7, SGA and low 1-minute Apgar score were risk factors (Table 3).
6. Risk factors for hyperglycemia
In order to evaluate the risk factors for hyperglycemia, univariate logistic regression analysis was performed using the same independent variables as those used for hypoglycemia. Risk of hyperglycemia within 24 hours decreased in those who had started feeding feeding (OR, 0.162; 95% CI, 0.040-0.662). The risk of hyperglycemia 2 to 7 days after birth increased in SGA infants and those with low 1-minute Apgar score (Table 4).
Discussion
Shortly after birth, the response of hormones regulating blood glucose levels is less sensitive because of the immaturity of cyclic adenosine monophosphate, a second messenger related to glucose metabolism. In addition, preterm neonates have a lower glycogen storage capacity than full-term neonates. Therefore, postnatal hypoglycemia is common in preterm neonates.
Hyperglycemia too is more common in preterm neonates than in full-term neonates. One of the reasons for this is a reduced ability to suppress endogenous glucose production in the body during glucose infusion. Another common mechanism responsible for hyperglycemia in preterm infants is the lack of insulin-sensitive tissues such as muscle and adipose tissue12). In addition, condition such as sepsis and necrotising enterocolitis can induce both hepatic and peripheral insulin resistance, which lead to stress induced hyperglycemia13). There is no consensus for definition of hypo- and hyperglycemia in neonates. In previous studies definition of hypoglycemia ranges from 1.7 to 2.6 mmol/L14). For defining hyperglycemia in preterm infants, cutoff value of 150 mg/dL or 180 mg/dL is commonly used 1516). In our study, hypo- and hyperglycemia was defined by values outside target blood glucose level used in our center.
The present study investigated blood glucose levels shortly after birth in preterm neonates, as well as associated risk factors for hypo- and hyperglycemia. The median blood glucose level was lowest 1 hour after birth, consistent with results from a study by Srinivasan et al.17) on blood glucose levels in full-term neonates. In that study, both the 5th- and 95th-percentile blood glucose levels were lowest from 10 to 14 hours18). However, in our study, the 5th-percentile blood glucose level was lowest at 2 hours after birth. This discrepancy is likely attributable to differences in glucose infusion as glucose was infused intravenously only when a neonate presented with hypoglycemia in Srinivasan et al.17)'s study, whereas intravenous glucose was given to all cases in our study. In the present study, prevalence of hypoglycemia peaked on day 2, consistent with the results obtained by Srinivasan et al.17). In the first 1 hour after birth, blood glucose level was the highest in GA<28 weeks group. This is explained by immaturity of beta cell enzymatic pathways and shortage of insulin sensitive tissue12).
In the present study, the incidence of hypoglycemia was higher in SGA infants than in infants with weights appropriate for GA (AGA). Concordantly, Lubchenco and Bard2) reported the incidences of hypoglycemia in preterm and full-term SGA neonates to be 67% and 25%, respectively, higher than those observed in AGA infants. Increased risk of hypoglycemia in SGA infants can be explained by decreased glycogen stores, increased insulin sensitivity and higher energy requirements19).
The incidence of hyperglycemia in the present study was 29.1%. Hays et al.20) previously reported an incidence of 60% within 7 days of birth. This difference may be accounted for by that study's inclusion of only infants with a birth weight <1 kg and a lower cutoff value of 8.33 mmol/L (150 mg/dL) for defining hyperglycemia.
In our study, 1-minute Apgar score was associated with both hypo- and hyperglycemia. This is in accordance with previous findings that neonates with perinatal distress are prone to both hypoglycemia21) and hyperglycemia22).
Both hypo- and hyperglycemia within 24 hours decreased in those who started feeding within 24 hours. On the contrary, in recent study of 203 SGA infants, feeding did not prevent hypoglycemia19). But this study included full term infants and investigated effect of early feeding defined as feeding before 4 hours after birth. The relationship of feeding with blood glucose level is not well known in preterm infants, and further investigation is required.
The major strengths of the present study lie in its singlecenter design, standardized blood glucose level measurement method, and relatively large sample size. Furthermore, we not only investigated the incidence of hypo- and hyperglycemia but also recorded detailed temporal changes in blood glucose levels.
Nevertheless, a limitation of this study is that the effect of intravenous glucose infusion was investigated only in conjunction with oral intake and was not analyzed in detail. Furthermore, we did not continuously measure blood glucose levels; instead, blood glucose levels were measured intermittently, usually once per day from 4 days onwards. Therefore, the overall incidence of hypo- and hyperglycemia may have been underestimated when compared to the incidence observed with continuous blood glucose monitoring. However, the prevalence of hypo- and hyperglycemia at each individual time point may have been overestimated, as blood glucose levels were measured more frequently in cases where hypo- and hyperglycemia developed.
In conclusion, hypo- and hyperglycemia is common in preterm infants and careful blood glucose level monitoring is required especially in SGA infants or those with low Apgar score. Early feeding could be beneficial for prevention of hypo- and hyperglycemia.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.