 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 18(4); 2013 > Article |
Abstract
Purpose
Serum glycated albumin (GA) has been recently used as another glycemic marker that reflects shorter term glycemic control than glycated hemoglobin (HbA1c). Insulin secretory function and glycemic fluctuation might be correlated with the ratio of GA to HbA1c (GA/HbA1c) in diabetic adult patients. This study investigated the association of GA and GA/HbA1c ratio with the levels of fasting C-peptide, fasting plasma glucose in type 1 and type 2 pediatric diabetes.
Methods
Total 50 cases from 42 patients were included. The subjects were classified into type 1 diabetes mellitus (T1DM) (n=30) and type 2 diabetes mellitus (T2DM) (n=20) group. The associations among HbA1c, GA, and GA/HbA1c ratio were examined. The relationship between the three glycemic indices and fasting glucose, fasting C-peptide were analyzed.
Results
Mean values of GA, the GA/HbA1c ratio were significantly higher in T1DM than T2DM. GA (r=0.532, P=0.001), HbA1c (r=0.519, P=0.002) and the GA/HbA1c ratio (r=0.409, P=0.016) were correlated with the fasting plasma glucose. Fasting C-peptide level arranged 4.22┬▒3.22 ng/mL in T2DM, which was significantly above the values in T1DM (0.26┬▒0.49 ng/mL). There were no significant correlation between HbA1c and fasting C-peptide level. However, GA and the GA/HbA1c ratio exhibited inverse correlations with fasting C-peptide level (r=-0.214, P=0.002; r=-0.516, P<0.001).
Glycated hemogloin (HbA1c) is widely used as the gold standard parameter of long-term glycemic control in diabetic patients. Mean HbA1c level is associated with the development and progression of diabetic complications. However, the use of HbA1c has some limitations. For instance, an integrated measurement of plasma glucose concentration during the previous 2 to 3 months is not suited to provide the evidence of earlier glycemic control and several conditions affecting a hemoglogin (Hb) metabolism such as anemia. Moreover it might be difficult that small-scaled values in HbA1c cannot estimate mean plasma glucose level1-5).
As another marker for monitoring glucose control, glycated albumin (GA) has been recently used. GA is the percentage of serum glycated albumin to total serum albumin and is not affected by the serum albumin level. Since a 50% reduction of the lifespan of albumin is 2-3 weeks, GA reflects shorter terms of glycemic control than HbA1c6-11).
Several studies for the relationship between levels of GA and HbA1c are recently reported in adult diabetic patients. Koga et al.12) revealed that the GA/HbA1c ratio in fulminant type 1 diabetes (FT1DM) could be a better marker for glycemic variability than HbA1c, which serum GA is higher in FT1DM than type 2 diabetes mellitus (T2DM), whereas HbA1c is significantly lower. The high GA/HbA1c ratio may reflect recent fluctuations in glucose levels and indicated recently increased postprandial glucose levels. There have also been accumulating evidences that the GA/HbA1c ratio is more associated with postprandial plasma glucose levels and insulin secretory function than HbA1c13,14). However, studies for GA and GA compared to HbA1c in pediatric diabetes have rarely been reported.
The present study investigated whether GA and the GA/HbA1c ratio are related to fasting C-peptide and fasting plasma glucose levels in pediatric diabetic patients, particularly comparing type 1 with type 2 diabetes.
Among the children with diabetes mellitus (DM) who visited the Department of Pediatrics at Inha University Hospital from October 2012 to September 2013, the subjects who underwent laboratory tests (HbA1c, GA, and fasting C-peptide) were included. Patients with liver disease, anemia were excluded. Total 50 cases from 42 patients were included in this study.
Clinical data was collected retrospectively from a review of medical records of patients. The subjects were classified into T1DM and T2DM group. Clinical and biochemical parameters on age, height, body weight, body mass index (BMI), BMI standard deviation score (SDS), DM duration, Hb, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum albumin, fasting glucose, fasting C-peptide, HbA1c, GA, and GA/HbA1c ratios were compared between two groups. BMI values were calculated using the 2007 growth reference tables for Korean children and adolescents of the Korean Pediatric Society and Korea Centers for Disease Control and Prevention15).
Serum glucose level was obtained using the hexokinase method with a Hitachi 7600-110 automatic analyzer (Hitachi Instruments Service, Tokyo, Japan). HbA1c level was measured with Tosoh HLC-723 G7 (Tosoh Co., Tokyo, Japan) by high-performance liquid chromatography. Serum GA level was determined by turbidimetric immunoassay using a Hitachi 7180 analyzer (Hitachi instruments service). Serum C-peptide level was measured using immunoradiometric assay with the C-peptide immunoradiometric assay (IRMA) kit (Beckman Coulter, Fullerton, CA, USA).
Correlations among HbA1c, serum GA, and GA/HbA1c ratio between groups were investigated. The relationship between three glycemic indices and fasting glucose, fasting C-peptide were analyzed.
All statistical analyses were performed using PASW ver. 18 (SPSS Inc., Chicago, IL, USA). All data are shown mean┬▒standard deviation. The independent samples t-test was used to compare clinical and biochemical parameters between the two groups. To evaluate the relationship between HbA1c and GA and GA/HbA1c ratio, linear regression analysis was used. A P-value of <0.05 was considered statistically significant.
A total of 50 cases (30 cases in T1DM group and 20 cases in T2DM group) ware analyzed (Table 1). The mean age was 12.03┬▒4.20 years in the T1DM group and 13.20┬▒1.73 years in the T2DM group (P=0.182). The height, weight, BMI, and BMI-SDS were significantly higher in the T2DM group than in the T1DM group (P<0.001). The average diabetes duration of T1DM group, 4.08┬▒4.56 years was longer compared with 1.34┬▒1.32 years in the T2DM (P=0.002). Mean fasting C-peptide level in T1DM group (0.26┬▒0.49 ng/mL) was lower than in the T2DM group (4.22┬▒3.22 ng/mL, P<0.001). There were no significant differences in fasting glucose and HbA1c levels between the two groups. The GA level and the GA/HbA1c ratio were significantly higher in the T1DM group (26.335%┬▒10.16%, 2.82%┬▒0.34%, respectively) than in the T2DM group (18.96%┬▒9.30%, 2.26%┬▒0.52%, respectively) (Fig. 1).
There were significant positive correlations between HbA1c and GA levels (r=0.945, P<0.001), and HbA1c and the GA/HbA1c ratio (r=0.625, P<0.001), and GA and the GA/HbA1c ratio (r=0.882, P<0.001). Furthermore, there was a strong significant correlation between HbA1c and GA (Fig. 2). There was no significant correlation between GA and blood Hb, GA and AST levels, GA and ALT levels, or GA and serum albumin levels.
There was a positive correlation between fasting glucose and each of the glycemic markers (HbA1c: r=0.519, P=0.002; GA: r=0.532, P=0.001; GA/HbA1c ratio: r=0.409, P=0.016) (Fig. 3).
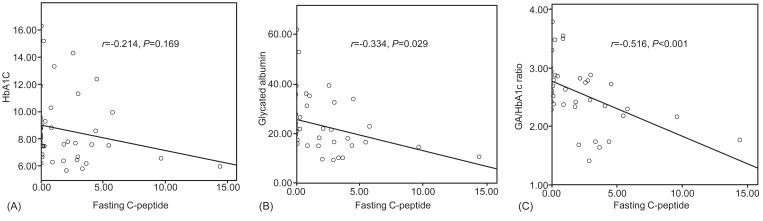
There was a negative correlation between fasting C-peptide and GA levels (r=-0.214, P=0.002), and C-peptide and GA/HbA1c ratio (r=-0.516, P<0.001) (Fig. 4.). But HbA1c was not correlated to fasting C-peptide (P=0.169).
Traditionally, HbA1c value has been used to measure long-term glycemic control over the preceding 2-3 months in DM patients. But recent studies have left areas of uncertainty about HbA1c: biological variability and clinical variability. Variability of Hb lifespan affects the HbA1c levels and the relationship of HbA1c monitoring to microvascular disease in T2DM is not strongly established1-5).
There are other reliable biomarkers for diabetes. The levels of glycated proteins reflect the degree of glycemic control during their life span, which glycation appears throughout the life span of Hb and serum proteins. Intermediate-term glycemic indicators are fructosamine and serum GA. Fructosamine assay is to measure the sum of all ketoamine linkages resulting from the glycation of circulating serum proteins. Fructosamine has been known to reflect the degree of hyperglycemia over 2-3 weeks in diabetes, whereas it is affected by serum albumin concentration16-18). GA is the similar to fructosamine excluding it is not affected by serum albumin level, because it is expressed as a percentage of serum albumin13). GA assay can get to be available method in commercial laboratories for clinical application in Korea.
In this study, the GA level and GA/HbA1c ratio were significantly higher in the T1DM group than the T2DM group comparing with no difference of HbA1c level between the two groups. Recent studies based on the continuous glucose monitoring system (CGMS) data have reported that GA better reflects glycemic fluctuation than HbA1c does11,19,20). CGMS data could not be collected in our study, but based on other study results, the difference of the GA/HbA1c ratio between the T1DM group and the T2DM group can be estimated short-term glycemic fluctuation of the T1DM group is more severe than that of the T2DM group.
The equation (Table 2) was devised to estimate the average plasma glucose levels using GA levels in order to easily evaluate glycemic control. To derive the relationship between HbA1c and estimated average glucose, the following formula was used: eAG (mg/dL)=28.7├ŚHbA1c-46.721). The correlated formula GA=4.0├ŚHbA1C-11.4, which was obtained from our result, was substituted for this (Fig. 2). This equation, eAG (mg/dL)=7.2├ŚGA+35.1, shows the corresponding values of GA, HbA1c, and eAG. GA 15% was corresponded to HbA1c 6.6% and eAG 143.1 mg/dL. The change in s magnitude of GA levels according to changes in glucose levels was larger than that of HbA1c values. Considering the point, GA can be considered more clinically useful for assessing the estimated glucose values.
This data showed serum GA levels and GA/HbA1c ratios were significantly correlated with HbA1c levels in pediatric diabetic patients. Otherwise, there was a negative correlation between fasting C-peptide and each glycemic marker, especially the correlation between fasting C-peptide and the GA/HbA1c ratio was more higher than that between fasting G-peptide and GA. Considering the fact that the fasting C-peptide level means endogenous insulin secretory function, our results showed the higher the insulin secretory function, the GA/HbA1c ratio declines further. A recent studies support GA level has more correlation with postprandial glucose concentration than mean plasma glucose level, comparing with HbA1c level11,22,23). The reasons why serum GA reflects postprandial glucose level better than HbA1c are still unknown. It can be considered that because of the higher insulin secretory function, smaller increase in postprandial glucose, the GA/HbA1c ratio decreases.
Some limitations of our study are as follows: This study had small number of cases and a limited cross-sectional method. Also we didn't collect the data of postprandial parameter such as plasma glucose, C-peptide level and therefore did not entirely verify the above finding.
Nonetheless, our study is meaningful as a first study about serum GA level and GA/HbA1c ratio in pediatric diabetes. It will be helpful for GA level and GA/HbA1c ratio to be used in the assessment of intermediate-term glycemic control in pediatric DM patients. The further studies are needed in pediatric diabetic patients.
References
1. Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995;18:440ŌĆō447. PMID: 7497851.


2. Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood 1982;59:1348ŌĆō1350. PMID: 7082831.


3. Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med 2004;21:657ŌĆō665. PMID: 15209755.


4. Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001;47:153ŌĆō163. PMID: 11159762.


5. Song MK, Bae JW, Kim YH, Chung S. Hemoglobin A1c in the screening of obesity-related diseases in children and adolescents. Ann Pediatr Endocrinol Metab 2012;17:92ŌĆō99.

6. Koga M, Hashimoto K, Murai J, Saito H, Mukai M, Ikegame K, et al. Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin Chim Acta 2011;412:253ŌĆō257. PMID: 20965159.


7. Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care 2010;33:509ŌĆō511. PMID: 20032277.


8. Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007;18:896ŌĆō903. PMID: 17267743.


9. Ai M, Otokozawa S, Schaefer EJ, Asztalos BF, Nakajima K, Shrader P, et al. Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin Chim Acta 2009;406:71ŌĆō74. PMID: 19465013.



10. Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 2002;324:61ŌĆō71. PMID: 12204426.


11. Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008;55:503ŌĆō507. PMID: 18445997.


12. Koga M, Murai J, Saito H, Kasayama S, Imagawa A, Hanafusa T, et al. Serum glycated albumin to haemoglobin A(1C) ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann Clin Biochem 2010;47(Pt 4):313ŌĆō317. PMID: 20516001.


13. Kim D, Kim KJ, Huh JH, Lee BW, Kang ES, Cha BS, et al. The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol (Oxf) 2012;77:679ŌĆō683. PMID: 22150917.


14. Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 2010;33:270ŌĆō272. PMID: 19846794.



15. Korea Centers for Disease Control and Prevention, Division of Chronic Disease Surveillance. Committee for the Development of Growth Standard for Korean Children and Adolescents. Korean Pediatric Society, Committee for School Health and Public Health Statistics. 2007 Korean children and adolescents growth standard (commentary for the development of 2007 growth chart). Cheongwon: Korea Centers for Disease Control and Prevention, Division of Chronic Disease Surveillance. 2007.
16. Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987;33:2153ŌĆō2163. PMID: 3319287.


17. Mosca A, Carenini A, Zoppi F, Carpinelli A, Banfi G, Ceriotti F, et al. Plasma protein glycation as measured by fructosamine assay. Clin Chem 1987;33:1141ŌĆō1146. PMID: 3594841.


18. Schleicher ED, Mayer R, Wagner EM, Gerbitz KD. Is serum fructosamine assay specific for determination of glycated serum protein? Clin Chem 1988;34:320ŌĆō323. PMID: 3124979.


19. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J 2010;57:135ŌĆō140. PMID: 19926921.


20. Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, Kang ES, et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 2011;48:167ŌĆō172. PMID: 21153482.


21. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473ŌĆō1478. PMID: 18540046.



22. Rhee EJ, Choi JH, Yoo SH, Bae JC, Kim WJ, Choi ES, et al. The association of unintentional changes in weight, body composition, and homeostasis model assessment index with glycemic progression in non-diabetic healthy subjects. Diabetes Metab J 2011;35:138ŌĆō148. PMID: 21738896.



23. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J 2011;35:107ŌĆō116. PMID: 21738892.



Fig.┬Ā1
Glycated hemoglobin (HbA1c) and serum glycated albumin (GA) and the GA/HbA1c ratio in patients with type 1 and type 2 diabetes mellitus (T1DM and T2DM).
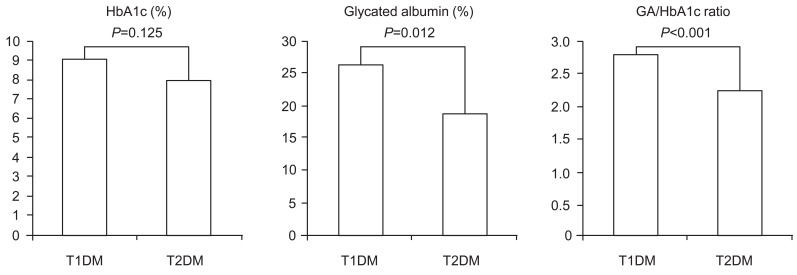
Fig.┬Ā2
Correlation between each glycemic markers. There were positive correlations between glycated hemoglobin (HbA1c) and glycated albumin (GA) (r=0.945, P<0.001), HbA1c and the GA/HbA1c ratio (r=0.625, P<0.001), and GA and the GA/HbA1c ratio (r=0.882, P<0.001).
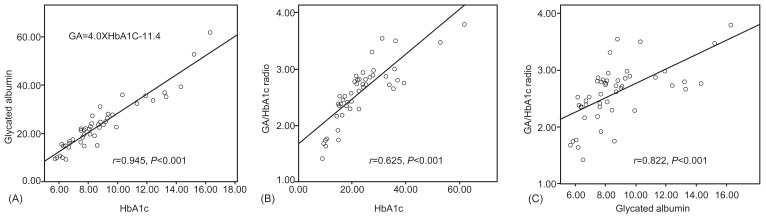
Fig.┬Ā3
Correlation between fasting glucose and each glycemic markers. There was a positive correlation between fasting glucose and each of the glycemic markers.
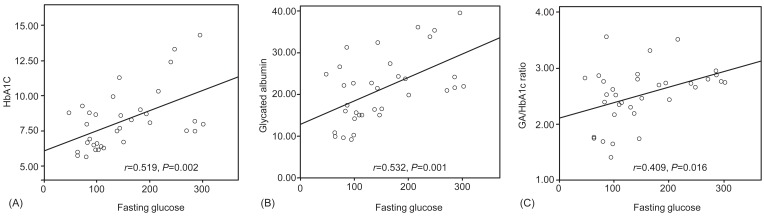
Fig.┬Ā4
Correlation between fasting C-peptide and each glycemic markers. There was a negative correlation between fasting C-peptide and glycated albumin (GA) levels (r=-0.334, P=0.029), and C-peptide and GA/HbA1c ratio (r=-0.516, P<0.001) (Fig. 3). But glycated hemoglobin (HbA1c) was not correlated to fasting C-peptide (r=-0.214, P=0.169).
Table┬Ā1.
Characteristics of the subjects and the laboratory data in type 1 and type 2 diabetic patients
Values are presented as mean┬▒SD or mean┬▒SD (range).
SD, standard deviation; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; BMI, body mass index; SDS, standard deviation score; DM duration, morbidity period for DM; AST, aspartate aminotransferase; ALT, alanine aminotransferase; FPG, fasting plasma glucose; GA/HbA1c, glycated albumin/glycated hemoglobin.
- TOOLS
-
METRICS

-
- 10 Crossref
- 8,236 View
- 153 Download
- Related articles in APEM





