Multicenter clinical trial of leuprolide acetate depot (Luphere depot 3.75 mg) for efficacy and safety in girls with central precocious puberty
Article information
Abstract
Purpose
We evaluated the efficacy, safety and psychological aspect of monthly administrations of the gonadotropin-releasing hormone agonists (GnRHa), leuprolide acetate depot (Luphere depot 3.75 mg), in patients with precocious puberty.
Methods
A total of 54 girls with central precocious puberty were administered with leuprolide acetate (Luphere depot 3.75 mg) every four weeks over 24 weeks. We evaluated the percentage of children exhibiting a suppressed luteinizing hormone (LH) response to GnRH (LH peak≤3 IU/L), peak LH/follicle stimulating hormone (FSH) ratio of GnRH stimulation test less than 1, change in bone age/chronologic age ratio, change in the Tanner stage and change in eating habit and psychological aspect.
Results
(1) The percentage of children exhibiting a suppressed LH response to GnRH, defined as an LH peak≤3 IU/L at 24 weeks was 96.3 % (52/54). (2) The percentage of children exhibiting peak LH/FSH ratio<1 at 24 weeks of the study was 94.4 % (51/54). (3) The ratio of bone age and chronological age significantly declined from 1.27±0.07 to 1.24±0.01 after the 6 months of the study. (4) The mean Tanner stage manifested a significant change 2.3±0.48 at baseline, down to 1.70±0.61 at 24 weeks. (5) Based on the questionnaires, the score for eating habits showed a significant change from the baseline 34.0±6.8 to 31.3±6.8. (6) The psychological assessment did not exhibit a significant difference except with scores for sociability, problem behavior total score and other problems.
Conclusion
The leuprolide 3.75 mg (Luphere depot) is useful and safety for treating children with central precocious puberty.
Introduction
Central precocious puberty (CPP) is defined by the development of gonadotropin dependent puberty occuring before the age of 8 years in girls and 9 years in boys1,2). CPP is caused by the premature reactivation of the hypothalamic-pituitary-gonadal-axis3). Gonadotropin stimulation induces the increases in sex steroid secretion responsible for the premature onset of somatic sexual characteristics, and is associated with growth spurt and accelerated skeletal maturation that compromises adult height4).
CPP is treated with gonadotropin-releasing hormone agonists (GnRHa), including leuprolide acetate, which has been the standard theraphy for more than 20 years5). Continuous exposure to GnRHa desensitizes pituitary gonadotroph receptors and suppresses luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion.
Efficacy of various GnRHa in CPP has been established6). Representative GnRHa are leuprorelin, goserelin, triptorelin, and histrelin. Leuprolide acetate depot (Luphere depot 3.75 mg) used in this study was leuprolide acetate formulation made by spray drying without methylene chlrolide and gelatin. The purpose of this study was to verify the effect of Luphere in comparison to GnRHa preciously used as well as, to evaluate changes in behaviors and eating habits.
Materials and methods
1. Study subjects
For this Korean multicenter study, 65 girls diagnosed with CPP and in treatment naïve states were recruited from 7 hospitals. The children were included on the basis of following inclusion criteria: clinical onset of pubertal development (breasts enlargement) before the chronological age (CA) of 8 years in girls, pubertal response of LH to GnRH stimulation test (peak≥5 IU/L), and bone age (BA)-CA≥1 year. BA was determined by Greulich and Pyle's standards, and pubertal stage was determined by Tanner stage7-9). To avoid the bias from several readings, one pediatric endocrinologist determined BA for all patients. Children exhibiting the following criteria were not included: gonadotropin-independent gonadal or adrenal sex steroid secretion; evaluative brain tumor requiring neurosurgery or brain irradiation; hypersensitiveness over investigative drug, synthetic GnRH or GnRH derivative; treatment with growth hormone; previous treatment with a GnRHa, medroxyprogesterone or cyproterone acetate; and concomitant disease likely to interfere with the study course.
2. Study design
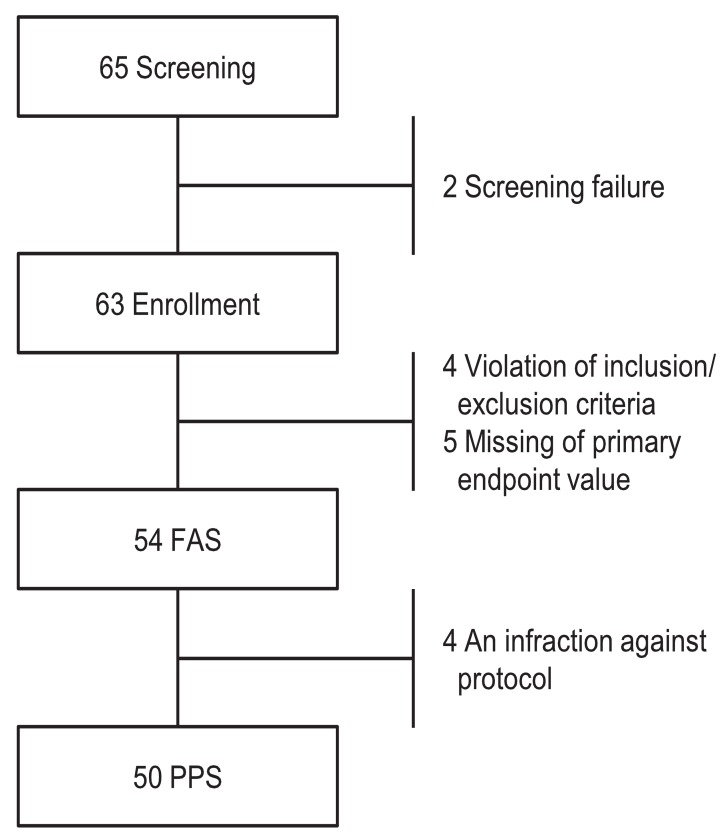
This was an open, multicenter, prospective study. This study included a screening period up to 4 weeks with treatment period of 24 weeks. Sixty-five subjects were screened in the study, and 63 were enrolled. Nine out of 63 subjects were excluded from the full analysis set (FAS) population (n=54). The reasons for the exclusion were "inclusion/exclusion criteria not met (n=4)" and "missing of primary efficacy endpoint value (n=5)" (Fig. 1). The data from the study was based on FAS.
3. Method
This study consisted of screening within 4 weeks and treatment for 24 weeks. GnRH stimulation test was done for inclusion/exclusion criteria for the initial visit (screening period). If a patient revisited the respective hospital within 4 weeks and met the inclusion/exclusion criteria, the drug was administered and was continued for 24 weeks throughout outpatient clinic visits (treatment period). During the treatment period of 24 weeks, the patients were subcutaneously injected with leuprolide acetate depot (Luphere depot 3.75 mg) in upper arms every 4 weeks for a total dosage by bodyweight; ≥14 kg to <20 kg, 1/3 vial; ≥20 kg to <30 kg, 2/3 vial; ≥30 kg, 1 vial. While the study was being conducted, drugs that can affect female hormone metabolism were not permitted to use because of possibly influence on evaluation variable of effectiveness.
The diagnosis of CPP was based on peak-stimulated LH concentration of at least 5 IU/L. GnRH stimulation test was followed 4 weeks after the final injection of leuprolide acetate depot (Luphere depot 3.75 mg).
The primary efficacy endpoint of the study was the percentage of children showing a suppressed LH response to GnRH, defined as an LH peak≤3 IU/L at week 24.
The secondary efficacy endpoints were the percentage of children showing LH peak/FSH peak ratio<1, change of BA/chronologic age ratio, and change of Tanner stage at week 24 of treatment.
In evaluation of primary efficacy, if the LH level was less than or equal to 3 IU/L at week 24 of the study, LH reaction was considered to be suppressed. Also, in the evaluation of secondary efficacy, if the LH peak/FSH peak ration was less than 1, LH reaction was considered to be suppressed. In addition, it was concluded that the suppression of LH reaction was well managed if there were lower levels and stages in BA/chronologic age ratio and Tanner stage.
The study of alteration in diet was conducted by a questionnaire on eating habits that subjects of the study completed at baseline visit and after 24 weeks. Questionnaire on eating habits was composed of 15 contents; Having breakfast on regularly, eating more than one bowl of rice, eating until feeling full, eating snacks more than eating regular meal (rice), eating again if there is a delicious food in spite of feeling full after meal, going on a binge eating, having a heavy dinner, having heavy snacks for late-night, eating lots of snacks (cracker candy, etc.), eating meat and greasy food oftentimes, drinking soda oftentimes, eating instant food or junk food oftentimes, eating lots of sweets. Each contents were assessed in 5 levels; always (daily, 5 point), often (5-6 days/wk, 4 point), usually (3-4 days/wk, 3 point), sometimes (1-2 days/wk, 2 point), never (0 days, 1 point). Maximum score for each questionnaire was 75 points. With low total scores, a patient was thought to have proper eating habits (only the first questionnaire of having breakfast regularly got 1 point for 'always' and got 5 points for 'never'). To assess changes in emotion and behavior, the Korea-Child Behavior Checklist (K-CBCL) was examined by dividing the subjects into two groups of under 6 years and 6 years of age or older. The K-CBCL is a standardized instrument used to obtain parental reports of competence and problem behavior of the child, and was developed to assess social adaptation, emotional, and behavior problems in children and adolescents, consists of 120 questions that constitute a social competence scale and a behavioral problem scale10).
4. Laboratory measurement
The hypothalamic-pituitary-gonadal axis was investigated by measuring plasma LH and FSH peak concentrations under GnRH (100 µg Relefact; Sanofi-Aventis, Frankfurt am main, Germany) stimulation. Basal serum samples were obtained prior to GnRH injection. Poststimulation samples for measurement of LH, FSH, and estradiol levels were acquired 30, 45, 60, and 90 minutes after injection. Serum LH and FSH levels were measured by immunoradiometric assay with analytical sensitivity of 0.2 and 0.1 IU/L, intra-assay coefficient of variation (CV) ranging from 1.4% to 3.9% and 1.1% to 2.0%, and inter-assay CV ranging from 3.4% to 8.0% and 2.4% to 4.4%, respectively (BioSource SA, Geneva, Switzerland). Estradiol levels were determined on radioimmunoassay with analytical sensitivity of 0.3 nmol/L, intra-assay CV ranging from 4.0% to 7.0% and inter-assay CV ranging from 4.2% to 8.1% (radioimmunoassay; Coat-A-Count, Diagnostic Products, Los Angeles, CA, USA).
5. Safety
Adverse events (AE), vital signs, clinical laboratory tests, subject symptoms, objective symptoms and injection site reactions were evaluated.
6. Statistics
As the primary endpoint evaluation of the trial, descriptive statistics were used for percentages of children with suppressed LH response on GnRH stimulation test at the end of the trial and a 95 percent two-sided confidence interval was suggested. And the percentage of children showing peak LH/FSH ratio <1 was using the same method. Wilcoxon signed rank test was implemented for the second effectiveness evaluation of the trial which is the change of BA/chronologic age ratio; change of tanner stage at 24 weeks of the study. Statistical analyses were performed with SAS 9.13 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the 54 enrolled patients were shown in Table 1. All of the subjects were girls and had clinical signs of puberty before the age of 8.
1. Gonadotropin suppression
The percentage of children showing suppressed LH response to GnRH, defined as an LH peak≤3 IU/L, was 96.3% (52/54) at 24 weeks of treatment (Table 2). And mean peak GnRH-stimulated LH value was 0.6±1.3 IU/L at week 24. The percentage of children showing LH peak/FSH peak ratio<1 was 94.4% at 24 weeks of treatment (n=51) (Table 2). And mean GnRH-stimulated LH peak/FSH peak ratio was 0.6±0.7 at week 24.
2. Changes in BA and Tanner stage
BA increased slowly after treatment. The ratio of BA and CA change 24 weeks after treatment significantly differed from values obtained at baseline from 1.27±0.07 to 1.24±0.01 (P < 0.0001) (Table 3). At the end of 24 weeks, clinical suppression of breast enlargement was observed compared with pretreatment Tanner stage of breast (P < 0.0001) (Table 3).
3. Change in eating habit scores
The score for eating habits from the baseline was compared with the scores at 24 weeks. Results are shown in Table 4. The decrease in the score was statistically significant (P = 0.0019). But change of the body mass index standard deviation score (SDS) was not statistically significant (Table 4).
4. Change in emotional and behavioral problems.
Psychological assessment was performed using the K-CBCL (n=54) during the initial study period and 24 weeks later. In the diagnostic and statistical manual of mental disorders-oriented subscale, the score for emotional problems, anxiety problems, somatic complaints, attention deficit hyperactivity disorder, oppositional defiant problems and conduct problems was not significant before and after the treatment.
In the problem behavior syndrome subscale, problem behavior total score and other problems were significantly lower at 24 weeks. However, problem behavior syndrome subscale, anxiety/depression, shrinking/depression, somatization, social immaturity, thought problem, attention problem, violation of regulation and offensive behavior did not change significantly during the treatment. In the problem behavior special subscale, scores for obsessive-compulsive symptoms, posttraumatic stress problems and sluggish cognitive tempo did not change significantly during treatment. In the adaptation scale profile subscale, the score for sociality was significantly higher at 24 weeks. However, the adaptive scale of total score and academic performance were not different before and after the treatment (Table 5).
5. Safety evaluation
After the agreement with the participation in the study, all subjects (n=63) who were administered with the investigational product at least once were synthetically judged through vital signs, lab tests, subjective symptoms, and objective symptoms. During the study, 31 out of 63 subjects experienced 57 AE. The most commonly reported drug reactions were pain, swelling, and urticaria at the injection site. Most events were mild, and there was no interruption in study procedures from these AE. Serious adverse event had not occurred during the study period.
Discussion
Leuproloride acetate is the first super active GnRHa and was originally synthesized by Fujino et al.11) at Takeda Chemical Industries. This analog, at acute doses, stimulates gonadotropin secretion by the pituitary and steroidogenesis in the genital organs. However, when administered chronically at a higher dose, it paradoxically produces antagonistic inhibitory effects on pituitary gonadotropin secretion12).
Luphere depot 3.75 mg is leuprolide acetate depot without methylene chloride and gelatin by spray drying. Even though the process of producing luphere depot differs from other leuprolide acetate present in current market, it has been shown already in previous studies that leuprolide acetate depot has a remedial effect on precocious puberty treatment13). Thus, this clinical trial is implemented to reconfirm the effectiveness and safety of Luphere depot 3.75 mg by spray drying.
In the study conducted by Neely et al.14) at nine United States (US) centers for 10 years, LH level and LH/FSH ratio were measured after treatment of leuprolide acetate (lupron) every 4 weeks for 49 girls and 10 boys with precocious puberty. Even though there was no measurement of suppression ratio to peak LH<3 IU/L like this study, the mean peak LH was suppressed to 0.7 IU/L (<0.5-3.15) at week 24 and the LH/FSH ratio was suppressed to 0.67. In our study, peak LH was 0.6±1.3 and peak LH/FSH ratio was 0.6±0.7 at week 24, so it was not much different from the journal.
According to studies of Carel et al.15) involving French children (40 girls and 9 boys), the percentage of children who showed a suppressed LH response to GnRH, defined as an LH peak≤3 IU/L was about 90 % at 6 months. This result was quite similar to our study. In the study conducted by Lee et al.16) at nine US centers, the changes in BA and CA of children injected with leuprolide acetate 3.75 mg (lupron) (subqutaneous injection at 4-week intervals for 12 months for 49 girls and 9 boys) at baseline and one year after the treatment revealed a difference that was decreased to 3.0 after one year treatment compared at baseline.
Although the 24-week-period of treatment was short, the BA/CA ratio showed significant change from pretreatment to post treatment (P < 0.0001). Also, regression of breast Tanner stage was observed at week 24. Even if there is no study regarding the change in eating habits after the GnRHa treatment in precocious puberty, various studies on pubertal status indicate that greater maturity in girls was associated with increased overall eating concerns17). In our study, total score of questionnaires on eating habits showed significant change from pretreatment score 34.0±6.8 to posttreatment score 31.3±6.8. In turn, this result can be seen as alerting an attention of the patients by checking the weight for every 4 weeks or education on proper diet. It is largely because patients usually do not have chance to see doctors and to be checked before the treatment.
There have been few studies on the change in psychological features associated with the GnRHa treatment in precocious puberty. In terms of psychological studies, Zheng et al.18) compared the psychological behavior of girls with idiopathic central precocious puberty (ICPP) before and after treatment by GnRHa. They used Raven's standard progressive matrices, Achenbach's CBCL, self-esteem scale (SES), and body-esteem scale (BES) to assess the psychological behavior in the ICPP girls before and after GnRHa treatment in one year, as well as in control group. They found that the SES and BES score in ICPP were significantly lower than those of controls (P < 0.05). The CBCL score in depressed, withdrawn, aggressive and somatic complaint assessment was significantly higher in ICPP group than those of control group. In our study, the problem behavior special subscale score of obsessive-compulsive symptoms, posttraumatic stress problems and sluggish cognitive tempo did not differ significantly during treatment. In the adaptation scale profile subscale, the score of sociality was significantly higher in the post treatment group (P < 0.05), but adaptive scale total score and academic performance did not differ. In the problem behavior syndrome subscale, problem behavior total score and other problems were significantly lower at week 24. However in the problem behavior syndrome subscale, anxiety/depression, shrinking/depression, somatization, social immaturity, thought problem, attention problem, violation of regulation and offensive behavior did not change significantly during treatment. The reason that the problem behavior total score and other problems were significantly low after the treatment was caused by the effects from the self-stress on body shape difference in peer group and treatment to suppress the increasing sexual activity. Also like eating habits, the regular visits to pediatric clinic and consultation with health providers every 4 weeks which was not made before treatment, would be helpful. Longer follow-up period is needed to provide sound conclusions on the effectiveness of improving psychological assessment.
The safety profile was similar for the other monthly depot formulations, with no new, unanticipated findings. Most of the adverse effects were injection site pain and swelling19).
The limitations of this study include the followings: a small sample size and short duration of treatment. Therefore, additional studies with more data and a longer follow-up are required in order to establish the effect of leuprolide acetate depot in patients with CPP.
In summary, leuprolide acetate depot (Luphere depot 3.75 mg) brought about consistent suppression of peak-stimulated LH. Positive changes in the eating habits score were observed. The psychological assessment did not yield any significant difference in the prepost treatment group except for scores associated with sociality, total score of problem behavior and other problems. Therefore, the present findings suggest that the leuprolide acetate depot 3.75 mg (Luphere depot) is useful and safety for treating children with precocious puberty.
Notes
No potential conflict of interest relevant to this article was reported.