 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(4); 2023 > Article |
|
Abstract
Purpose
Methods
Results
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: SHL, HGL, EMY, CJK; Data curation: SHL, HGL, EMY, CJK; Formal analysis: SHL, HGL, CJK; Funding acquisition: SHL, CJK; Methodology: SHL, HGL, EMY, CJK; Project administration: SHL, HGL, EMY, CJK; Visualization: SHL, EMY, CJK; Writing - original draft: SHL; Writing - review & editing: SHL, HGL, EMY, CJK
Fig. 1.
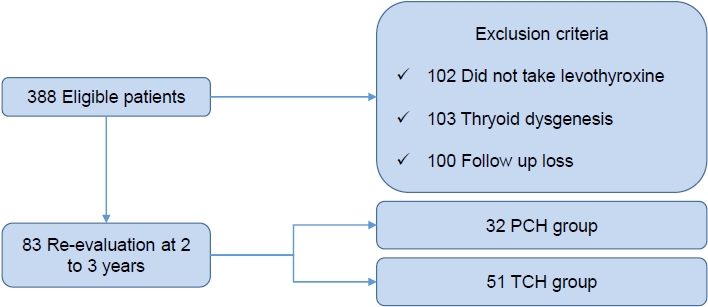
Fig. 2.
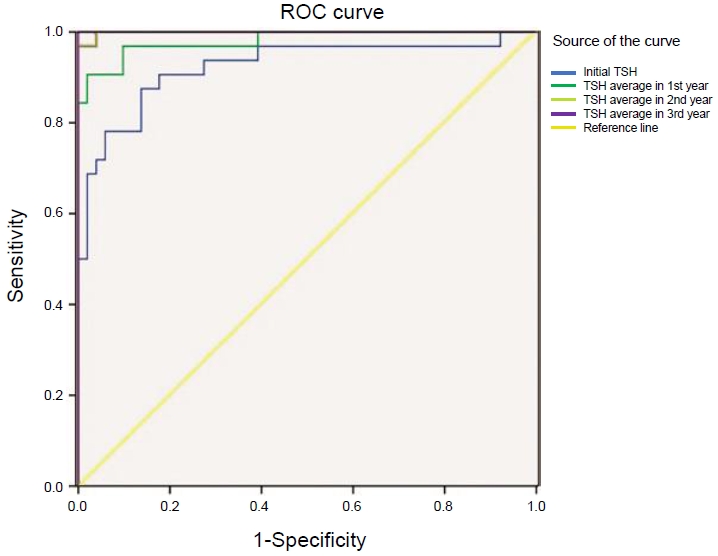
Fig. 3.
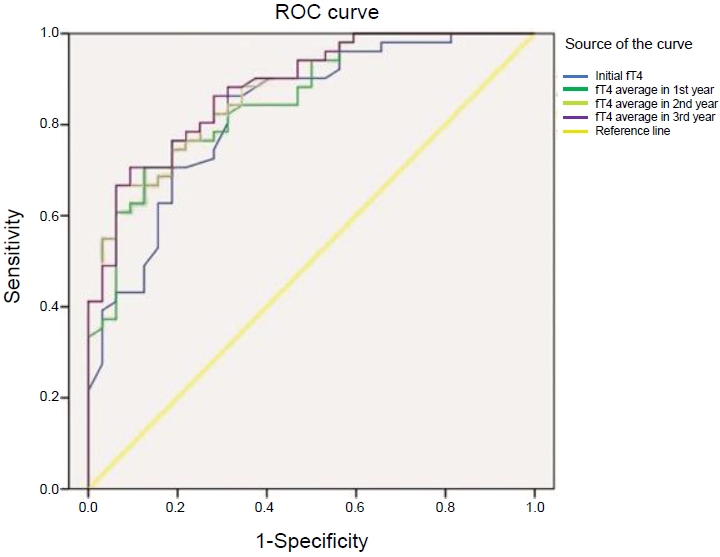
Table 1.
| Characteristic | PCH (n=32) | TCH (n=51) | P-value |
|---|---|---|---|
| Sex, male:female | 17:15 | 34:17 | 0.222 |
| TSH (μIU/mL) | 71.21±44.94 | 50.41±41.82 | 0.060 |
| fT4 (ng/dL) | 0.74±0.42 | 0.94±1.09 | 0.380 |
| Gestational age (wk) | 37.8±4.5 | 36.7±4.3 | 0.339 |
| Birth weight (kg) | 2.9±0.9 | 2.8±0.9 | 0.529 |
| SGA (kg) | 3.0±0.7 | 1.9±1.0 | 0.237 |
| AGA (kg) | 2.8±0.9 | 2.8±0.9 | 0.941 |
| Feeding | 1.4±0.6 | 1.4±0.7 | 0.782 |
| Levothyroxine dose (μg/kg) | |||
| Initial | 9.83±3.45 | 8.99±2.48 | 0.242 |
| 1 Years | 3.32±0.85 | 2.81±0.91 | 0.019* |
| 2 Years | 3.11±0.99 | 2.34±0.87 | 0.001* |
Values are presented as mean±standard deviation and number.
P-value using a Student t-test between the 2 groups.
PCH, permanent congenital hypothyroidism; TCH, transient congenital hypothyroidism; TSH, thyroid-stimulating hormone; fT4, free thyroxine; SGA, small for gestational age; AGA, appropriate for gestational age.
Table 2.
| Variable | Optimal cutoff value (μIU/mL) | Sensitivity | Specificity | P-value |
|---|---|---|---|---|
| Initial TSH | 7.79 | 87.5% | 86.3% | 0.001* |
| Mean TSH in 1st year | 9.76 | 90.6% | 98.0% | 0.001* |
| Mean TSH in 2nd year | 9.87 | 96.9% | 100.0% | 0.001* |
| Mean TSH in 3rd year | 9.05 | 100.0% | 100.0% | 0.001* |
| Initial fT4 | 1.07 | 86.3% | 68.7% | 0.001* |
| Mean fT4 in 1st year | 1.18 | 70.6% | 87.5% | 0.001* |
| Mean fT4 in 2nd year | 1.20 | 66.7% | 93.7% | 0.001* |
| Mean fT4 in 3rd year | 1.20 | 66.7% | 93.7% | 0.001* |







