|
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(4); 2023 > Article |
|
See commentary "Commentary on "Glycemic control and complications of type 2 diabetes mellitus in children and adolescents during the COVID-19 outbreak"" in Volume 28 on page 233.
Abstract
Purpose
This study aimed to investigate the impact of coronavirus disease 2019 (COVID-19) on type 2 diabetes mellitus (T2DM) in children and adolescents.
Methods
Children and adolescents diagnosed with T2DM who visited the Korea University Hospital in 2019 and 2020 were retrospectively analyzed, including changes in body mass index (BMI)-standard deviation score (SDS), glycated hemoglobin (HbA1c), diabetes complications, and diabetes management from 2019 to 2020.
Results
Patient mean age and disease duration were 15.48±2.15 and 2.56±1.51 years, respectively. Obese patients accounted for 70.6% of the study population. From 2019 to 2020, mean BMI-SDS (2.21±1.25 vs. 2.35±1.43, P=0.044), HbA1c level (6.5%±2.72% vs. 7.3%±3.70%, P<0.001), blood pressure (BP), total cholesterol, and non–high-density lipoprotein cholesterol level in all patients increased significantly. Obesity was an independent predictor of increased HbA1c (95% confidence interval, 1.071–50.384; P=0.042). HbA1c levels did not increase significantly in nonobese patients, whereas HbA1c (6.45%±2.30% vs. 7.20%±3.05%, P<0.001), BMI-SDS (2.88±0.75 vs. 3.08±0.98, P=0.045), diastolic BP (P=0.037), and total cholesterol values (P=0.019) increased in obese patients in 2020 compared to 2019.
Conclusions
During the COVID-19 outbreak, glycemic control and diabetic complications worsened in children and adolescents with T2DM, particularly in obese patients. Close monitoring for glycemic control and diabetic complications is necessary in children and adolescents with T2DM, especially those with obesity.
· During the coronavirus disease-2019 outbreak, glycemic control and diabetic complications are worsen in children and adolescents with type 2 diabetes mellitus (T2DM). Prominent aggravation of glycemic control was observed especially in obese patients.
· We need to closely monitor glycemic control and diabetic complications in children and adolescents with T2DM, especially in obese patients.
Since it was first reported in December 2019, coronavirus disease-2019 (COVID-19) has caused pandemic infections. Global containment measures have attempted to stop the spread of this virus [1]. Worldwide, children and adolescents were prohibited from going to school or participating in outdoor activities to slow the spread of the virus [2,3]. Schools were completely closed for approximately 3 months after initiation of social distancing (March, 2020). In Korea, from May 20, 2020, schools were allowed to open sequentially for each grade, by the Korean Ministry of Education [4].
The prevalence of pediatric obesity has more than doubled worldwide since 1980 [5]. According to 1 population-based study, the global age-standardized obesity prevalence in 2016 was 7.8% in boys and 5.6% in girls, which were approximately 8 times higher than in 1975 [6]. In South Korea, the prevalence of pediatric obesity increased from 6.55% in 2009 to 11.64% in 2018 [7]. The obesity prevalence in children and adolescents was 21.8% and 15.7% in the United States (US) and China, respectively, and the prevalence of obesity continues to increase in children, adolescents, and young adults, especially during the COVID-19 outbreak [8,9].
In a study of nearly 300,000 children, the overall prevalence of obesity increased from 13.7% (June 2019 to December 2019) to 15.4% (June 2020 to December 2020) in the US [8]. In North Africa, patients' mean self-reported weight and body mass index (BMI) significantly increased by 1.43 kg and 0.84 kg/m2, respectively, from April 2020–May 2020 to July 20200August 2020 [10]. According to Chinese data, the obesity prevalence in youths increased from 10.5% to 12.9% from 2019 to 2020 [9]. In South Korea, Gwag et al. [4] reported that the BMI-standard deviation score (SDS) and the proportion of overweight and obese patients had gradually increased during the COVID-19 pandemic. Roh et al. [11] also reported that the rates of being overweight or obese in new outpatients who visited growth clinics from May 2019 to July 2019 were 25.3% for girls and 23.3% for boys. The corresponding rates for the same period in 2020 (N=201; 153 girls, 48 boys) were 31.4% for girls and 45.8% for boys. Kang et al. [12] reported that the duration of school closure is significantly associated with an increased BMI in children during the COVID-19 pandemic.
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by hyperglycemia with insulin resistance. Obesity is the main risk factor for numerous metabolic diseases including T2DM, dyslipidemia, hypertension, and nonalcoholic fatty liver disease (NAFLD). More than 90% of patients with T2DM are overweight or obese [13,14]. Many obese adolescents stay obese into adulthood and obesity-related comorbidities begin as early as childhood, which increases morbidity and mortality due to cardiovascular, metabolic, or oncological illnesses [15].
Until 30 years ago, T2DM rarely occurred in pediatric populations [16], but the incidence of T2DM in children and adolescents has increased worldwide over the years, coinciding with the increase in obesity [17,18]. In South Korea, the proportion of T2DM among adolescents with diabetes increased 4-fold from 5.3% in 1990 to 21.0% in 2000 [19]. According to another study based on the Korean National Health Insurance Service database, the prevalence of T2DM per 10,000 people increased from 2.57 in 2002 to 11.41 in 2016 in male individuals, and from 1.96 in 2002 to 8.63 in 2016 in female individuals [20]. Childhood-onset T2DM is associated with a greater risk of macrovascular and microvascular complications such as retinopathy, neuropathy, and nephropathy compared with childhood-onset type-1 diabetes mellitus (T1DM) [21]. Strict glycemic control is important to prevent such diabetic complications [22].
Previous studies compared changes in the incidences of T1DM and T2DM in pediatric patients during the COVID-19 pandemic and analyzed the clinical features of patients with T2DM newly diagnosed during the COVID-19 pandemic [23,24]. Other studies also showed that the number of pediatric patients newly diagnosed with T2DM increased during the COVID-19 pandemic [25,26], but studies on how the COVID-19 pandemic affected glycemic control or complications in pediatric patients with T2DM are insufficient. Therefore, this study aimed to investigate the impact of the COVID-19 pandemic on T2DM, especially on glycemic control and diabetic complications in children and adolescents.
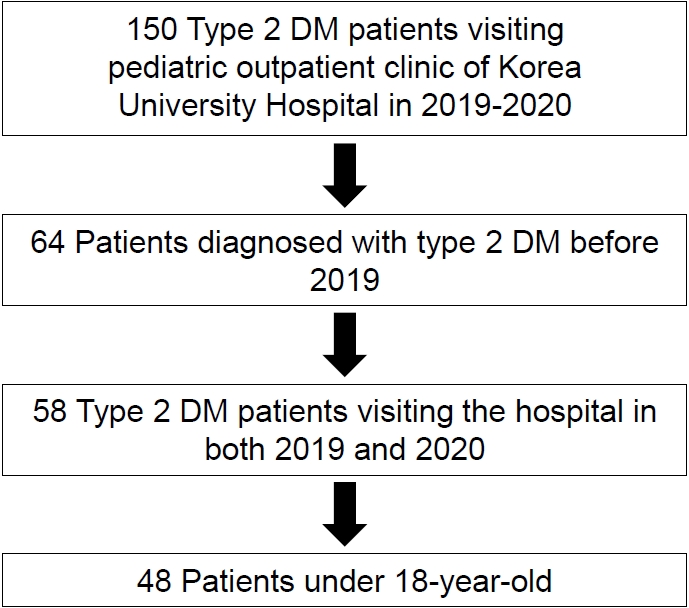
The COVID-19 pandemic started after January 2020, in Korea, when the first COVID-19 case was confirmed. Subsequently, the number of cases increased rapidly [4]. A retrospective case analysis of children and adolescents diagnosed with T2DM who visited the pediatric endocrinology clinic of the Korea University Hospital in 2019 and 2020 was performed. In total, 150 patients were initially enrolled. Children who fulfilled all of the following inclusion criteria were included: (1) patients diagnosed with T2DM before 2019 who have had the disease for >6 months (N=64); (2) patients who visited the hospital and underwent anthropometric and laboratory tests in both 2019 and 2020 (N=58); and; (3) patients younger than 18 years old (N=48) (Fig. 1).
We reviewed patients' medical records and analyzed the following parameters: age at diagnosis, age at each visit, sex, anthropometric findings, and physical findings. SDSs for weight, height, and BMI were calculated for each patient. Obesity was defined as a BMI above the 95th percentile or higher by the 2017 Korean national growth charts for children [27]. Hypertension was defined as systolic or diastolic blood pressure (BP) in the 95th percentile or higher according to age and sex, or 130/80mmHg or higher in children aged 13 years or older [28].
We analyzed patients' glycated hemoglobin (HbA1c) level, liver function studies, and lipid profiles. Blood samples were collected from the antecubital vein after an 8-hour fasting and were subsequently processed and immediately refrigerated. HbA1c levels were measured by high-performance liquid chromatography (Variant II, Variant Turbo; Bio-Rad, Hercules, CA, USA). Serum levels of total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) were measured using the Advia 1650/2400 (Siemens, Munich, Germany) in the 2007 survey, and the Hitachi Automatic Analyzer 7600/7600-210 (Hitachi, Ibaraki, Japan) in the 2008–2018 surveys. Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula (LDL-C=total cholesterol–[HDL-C+(TG/5)]) [29]. Serum aspartate aminotransferase and alanine aminotransferase (ALT) were measured by ultraviolet spectrophotometry without the pyridoxal-5ʹ-phosphate method using commercially available kits (Pureauto S ALT; Daiichi Pure Chemicals, Tokyo, Japan).
Dyslipidemia was defined as total cholesterol level ≥200 mg/dL, LDL-C level ≥130 mg/dL, HDL-C level <40 mg/dL, and non-HDL-C level ≥145 mg/dL, and TG level ≥100 mg/dL (0–9 years)/130 mg/dL (10–19 years) [30,31]. If any of the aforementioned criteria were satisfied, the patient was diagnosed as having dyslipidemia. NAFLD was defined as ALT level above the upper normal range (26 U/L for male individuals and 22 U/L for female individuals) and ultrasonographically confirmed hepatic fat accumulation [32,33].
Diabetic nephropathy was defined as microalbuminuria (urine microalbumin level >30 mg/day) from a 24-hour urine test [34]. Diabetic peripheral neuropathy was confirmed when there were abnormal findings on electromyography regardless of neuropathic symptoms. The number of hospital visits each year and changes in therapy, such as dose of medication and insulin treatment, were also examined.
When the patient visited the clinic more than once a year, the average anthropometric and laboratory findings were calculated and documented.
Continuous variables are expressed as means±standard deviations. Categorical variables are described as percentages and proportions. The paired t-test was used to analyze the normally distributed continuous variables, whereas the Wilcoxon signed-rank was used to analyze variables that were not normally distributed. The McNemar test was used for prevalence comparisons before and after the COVID-19 outbreak. Multivariable logistic regression analysis was used to identify risk factors for HbA1c deterioration. Statistical analysis was performed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). A P-value <0.05 was considered statistically significant for all tests.
Patients' baseline characteristics are presented in Table 1. Patient mean age was 15.48±2.15 years, and mean disease duration was 2.56±1.51 years. The mean weight-SDS and height-SDS were 2.33±1.31 and 1.98±1.23, respectively. The BMI and BMI-SDS were calculated for 34 patients using weight and height measurement records. Obese patients accounted for 70.6% of the patients. The mean BMI-SDS was 2.32±1.26. Hypertension, dyslipidemia, and NAFLD were diagnosed in 38.6%, 38.3%, and 44.6% of patients, respectively. In our study, 7 of 46 patients (15.2%) were diagnosed with diabetic nephropathy, and none of the patients were diagnosed with diabetic peripheral neuropathy before the COVID-19 pandemic. Overall, 45.8% were treated with oral hypoglycemic agents (metformin, N=21; sulfonylurea, N=1) without insulin. The remaining patients were using both insulin and oral hypoglycemic agents.
Comparisons of glycemic control and diabetic complications in patients between 2019 and 2020 are shown in Table 2. Mean weight-SDS of the study participants was higher in 2019 than in 2020 without statistical significance (2.33±1.31 vs. 2.20±1.24, P=0.210). Mean BMI-SDS was higher in 2020 than before the COVID-19 pandemic (2.21±1.25 vs. 2.35±1.43, P=0.044). From 2019 to 2020, patients' mean HbA1c level also increased (6.50±2.72 vs. 7.30±3.70, P<0.001). Patients' mean values of systolic and diastolic BP, total cholesterol, and non-HDL cholesterol were higher in 2020 than in 2019. However, values of TG, LDL-C, liver function tests, and 24-hour urine microalbumin tests were not significantly different between 2019 and 2020 in the study group. During the COVID-19 pandemic, the number of hospital visits by patients decreased from 4.50±1.00 to 4.00±3.00. However, the number of patients receiving insulin treatment and total insulin dose used increased, although not with statistical significance. In multivariable logistic regression analysis conducted to investigate the risk factors of increased HbA1c, obesity was an independent predictor of increased HbA1c levels (95% confidence interval, 1.071–50.384; P=0.042) (Table 3).
Depending on their initial weight status, patients presented with varying degrees of glycemic control and diabetic complications. In nonobese patients, mean HbA1c level did not significantly increase during the COVID-19 outbreak. Diabetic complications and treatment options showed no significant changes in the nonobese group. However, in obese patients, mean BMI-SDS and HbA1c level increased during the COVID-19 outbreak (2.88±0.75 vs. 3.08±0.98, P=0.045 and 6.45±2.30 vs. 7.20±3.05, P<0.001, respectively). Mean diastolic BP and total cholesterol levels in obese patients were also higher in 2020 than in 2019. The number of patients receiving insulin treatment and the total insulin dose used by patients increased, although these findings were not statistically significant (Tables 4, 5).
This study demonstrated t hat glycemic control and complications of T2DM in children and adolescents worsened during the COVID-19 pandemic period, especially in obese patients. From 2019 to 2020, mean values of BMI-SDS, HbA1c, BP, total cholesterol, and non-HDL cholesterol in all patients increased, and this trend was stronger in patients who were previously obese.
The COVID-19 pandemic has persisted for over 2 years, making a return to regular life difficult [35]. Health problems caused by COVID-19, such as aggravation of obesity and diabetes, are expected to continue [26]. The prevalence of obesity in children and adults increased during the COVID-19 pandemic. However, few studies have dealt with changes in existing pediatric patients with T2DM during the COVID-19 pandemic.
Mean BMI-SDS of 34 patients with height and weight data was 2.32, among whom 70.6% were obese, and 85.3% were overweight or obese. These ratios are similar to previous reports [36].
In our study, glycemic control and BMI-SDS worsened in children and adolescents as well as in adults during the COVID-19 pandemic. In one retrospective study of 128 adult patients with T2DM, participants' weight and BMI increased significantly during the lockdown period (between May 15, 2020 and June 30, 2020). Fasting plasma glucose and HbA1c levels also increased, with weight gain having a direct association with an increase in HbA1c levels [37]. In another single center study, Onmez et al. [38] reported that HbA1c levels increased after a 75-day lockdown from 7.67% to 8.11%, although this was statistically not significant.
HbA1c levels significantly increased only in obese patients, which was thought to be due to the more pronounced increase in BMI-SDS in previously obese patients. Whether the BMI-SDS increased further in obese patients during the COVID-19 outbreak is controversial. In one cohort study in the US, mean BMI increased during the COVID-19 pandemic and this increase was prominent in children with pre-existing obesity [39]. However, according to a Chinese study of 72,175 children aged 8–12 years, the obesity prevalence increased from 2019 to 2020, but the increase in BMI-SDS was greater in underweight participants [40]. Although our study did not analyze the factors associated with increased BMI-SDS, the reasons for increases in BMI-SDS during the COVID-19 pandemic in previously obese pediatric patients might have been due to deterioration of dietary habits or increases in sedentary time, according to previous studies. Diets high in sugar and fat and decreased physical activity due to social distancing are thought to be correlated with increasing obesity during the COVID-19 pandemic [41,42].
Among various T2DM complications, hypertension and dyslipidemia deteriorated during the COVID-19 pandemic in our study. As in pediatric patients, there remains a lack of research on adults to reveal correlations between diabetic complications and the COVID-19 pandemic. In one multi-center study of 283 diabetic patients in Turkey, the number of patients who had controlled BP was significantly decreased and the number of patients with neuropathic complaints and severe dyslipidemia significantly increased during the pandemic period [43]. The lack of significant differences in diabetic nephropathy between 2019 and 2020 in pediatric patients with T2DM could have been because microvascular complications take a long time to develop.
Our study has several limitations. First, this was a retrospective study limited to the South Korean population. Second, height and weight were measured in only 34 of 48 patients in 2019 and 2020. Moreover, fasting TG levels were measured in only 12 patients. Third, NAFLD should be diagnosed by liver biopsy, but biopsy was not performed in this study. Additionally, confounding factors such as physical activity and nutrition were not considered. Our study only described changes in diabetic patients in 2019 and 2020, just after the COVID-19 pandemic. Thus, a study comparing patients over longer periods is needed. Also, studies on various factors such as nutrients and physical activity that contribute to glycemic control and complications in children and adolescents with T2DM should be conducted.
In conclusion, during the COVID-19 outbreak, glycemic control and diabetic complications worsened in children and adolescents with T2DM. This tendency was prominent in obese patients. Therefore, close monitoring for glycemic control and diabetic complications is necessary in children and adolescents with T2DM, especially those with obesity.
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Table 1.
Baseline characteristics of patients (N=48)
Table 2.
Comparison of glycemic control and complications of T2DM between 2019 and 2020 (N=48)
| Variable | 2019 | 2020 | P-value |
|---|---|---|---|
| Weight-SDS | 2.33±1.31 | 2.20±1.24 | 0.210† |
| Height-SDS (N=31) | 1.98±1.23 | 1.85±1.29 | 0.205† |
| BMI-SDS (N=31) | 2.21±1.25 | 2.35±1.43 | 0.044† |
| Glycated hemoglobin (%) | 6.50±2.72 | 7.30±3.70 | 0.000‡ |
| Systolic BP (mm/Hg) (N=44) | 125.00±19.00 | 132.00±19.50 | 0.016‡ |
| Diastolic BP (mm/Hg) (N=44) | 70.50±11.75 | 79.00±16.75 | 0.000‡ |
| AST (U/L) (N=47) | 29.00±27.00 | 28.00±37.25 | 0.274‡ |
| ALT (U/L) (N=47) | 44.00±54.00 | 51.00±64.25 | 0.302‡ |
| Total cholesterol (mg/dL) (N=47) | 171.00±35.00 | 181.00±48.50 | 0.002‡ |
| Non-HDL cholesterol (mg/dL) (N=47) | 129.00±48.50 | 132.50±52.50 | 0.020‡ |
| Triglyceride (mg/dL) (N=12) | 115.00±37.00 | 118.50±39.50 | 0.180‡ |
| LDL cholesterol (mg/dL) (N=12) | 106.00±30.50 | 120.00±42.50 | 0.196‡ |
| 24-Hour urine microalbumin (mg/day) (N=33) | 8.90±17.60 | 8.35±15.78 | 0.694‡ |
| Patients with nephropathy | 7 (14.5) | 8 (16.7) | 0.302§ |
| No. of times visiting a hospital annually | 4.50±1.00 | 4.00±3.00 | 0.472‡ |
| Patients treated with oral hypoglycemic agent and insulin | 25 (52.1) | 28 (58.3) | 0.375§ |
| Total insulin dose used in patients (IU/kg/day) | 0.28±0.38 | 0.30±0.53 | 0.080‡ |
Table 3.
Univariable and multivariable logistic regression analysis for increased glycated hemoglobin (N=48)
Table 4.
Comparison of glycemic control and complications of T2DM according to the initial weight status of patients (normal and overweight patients, N=10)
| Variable | 2019 | 2020 | P-value |
|---|---|---|---|
| Weight-SDS | 0.81±1.17 | 0.77±1.17 | 0.602† |
| Height-SDS | 1.65±1.02 | 1.63±0.98 | 0.532† |
| BMI-SDS | 0.79±0.79 | 0.82±0.88 | 0.728† |
| Glycated hemoglobin (%) | 6.25±3.28 | 6.55±3.20 | 0.502‡ |
| Systolic BP (mm/Hg) | 116.00±13.75 | 117.00±13.50 | 0.878‡ |
| Diastolic BP (mm/Hg) | 67.5±15.25 | 69.5±49.00 | 0.386‡ |
| AST (U/L) (N=9) | 21.00±44.00 | 21.00±13.25 | 0.528‡ |
| ALT (U/L) (N=9) | 23.00±41.00 | 20.50±33.25 | 0.859‡ |
| Total cholesterol (mg/dL) (N=9) | 159.00±29.50 | 177.50±70.25 | 0.066‡ |
| Non-HDL cholesterol (mg/dL) (N=9) | 94.00±31.00 | 112.00±64.00 | 0.400‡ |
| 24-Hour urine microalbumin (mg/day) (N=5) | 11.60±48.70 | 9.70±15.18 | 0.176‡ |
| No. of times visiting a hospital annually | 4.50±3.00 | 4.00±3.00 | 0.500‡ |
| Patients treated with oral hypoglycemic agent and insulin | 2 (20) | 2 (20) | 1.000§ |
| Total insulin dose used in patients (IU/kg/day) (N=2) | 0.57 | 0.75 | 0.655‡ |
Table 5.
Comparison of glycemic control and complications of T2DM according to the initial weight status of patients (obese patients, N=24)
| Variable | 2019 | 2020 | P-value |
|---|---|---|---|
| Weight-SDS | 2.96±0.97 | 2.80±0.85 | 0.364† |
| Height-SDS (N=21) | 2.12±1.10 | 2.03±1.08 | 0.109† |
| BMI-SDS (N=21) | 2.88±0.75 | 3.08±0.98 | 0.045† |
| Glycated hemoglobin (%) | 6.45±2.30 | 7.2±3.05 | 0.000‡ |
| Systolic BP (mm/Hg) (N=22) | 128.00±16.50 | 132.50±20.00 | 0.101‡ |
| Diastolic BP (mm/Hg) (N=22) | 72.50±15.50 | 79.50±14.75 | 0.037‡ |
| AST (U/L) | 31.50±34.00 | 45.50±48.00 | 0.568‡ |
| ALT (U/L) | 53.00±57.25 | 74.00±79.00 | 0.475‡ |
| Total cholesterol (mg/dL) (N=23) | 174.00±29.00 | 185.5±38.00 | 0.019‡ |
| Non-HDL cholesterol (mg/dL) (N=23) | 134.00±35.00 | 139.00±35.00 | 0.079‡ |
| 24-Hour urine microalbumin (mg/day) (N=17) | 9.60±17.40 | 8.90±27.97 | 0.811‡ |
| No. of times visiting a hospital annually | 5.00±2.00 | 5.00±2.75 | 0.401‡ |
| Patients treated with oral hypoglycemic agent and insulin | 14 (58.3) | 16 (66.7) | 0.500§ |
| Total insulin dose used in patients (IU/kg/day) | 0.26±0.40 | 0.29±0.54 | 0.432‡ |
References
1. Du Q, Zhang D, Hu W, Li X, Xia Q, Wen T, et al. Nosocomial infection of COVID19: a new challenge for healthcare professionals (Review). Int J Mol Med 2021;47:31.



2. Panovska-Griffiths J, Kerr CC, Stuart RM, Mistry D, Klein DJ, Viner RM, et al. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: a modelling study. Lancet Child Adolesc Health 2020;4:817–27.



3. Xiang M, Zhang Z, Kuwahara K. Impact of COVID-19 pandemic on children and adolescents' lifestyle behavior larger than expected. Prog Cardiovasc Dis 2020;63:531–2.



4. Gwag SH, Oh YR, Ha JW, Kang E, Nam HK, Lee Y, et al. Weight changes of children in 1 year during COVID-19 pandemic. J Pediatr Endocrinol Metab 2022;35:297–302.


5. Weihrauch-Bluher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019;92:147–52.


6. Song K, Jeon S, Lee HS, Choi HS, Suh J, Kwon A, et al. Trends of dyslipidemia in Korean youth according to sex and body mass index: based on the Korea National Health and Nutrition Examination Survey (2007-2018). J Pediatr 2021;237:71–8.e5.


7. Song K, Park G, Lee HS, Lee M, Lee HI, Ahn J, et al. Trends in prediabetes and non-alcoholic fatty liver disease associated with abdominal obesity among Korean children and adolescents: based on the Korea National Health and Nutrition Examination Survey between 2009 and 2018. Biomedicines 2022;10:584.



8. Jenssen BP, Kelly MK, Powell M, Bouchelle Z, Mayne SL, Fiks AG. COVID-19 and changes in child obesity. Pediatrics 2021;147:e2021050123.



9. Yang S, Guo B, Ao L, Yang C, Zhang L, Zhou J, et al. Obesity and activity patterns before and during COVID-19 lockdown among youths in China. Clinc Obes 2020;10:e12416.
10. Benmerzoug M, Djoudi B, Debbache A, Harbouche A, Dehmani ID, Djekkoun N, et al. Impact of COVID-19 lockdown on children's health in North Africa. Matern Child Health J 2022;26:1701–8.




11. Roh SM, Eun BW, Seo JY. Does coronavirus disease 2019 affect body mass index of children and adolescents who visited a growth clinic in South Korea?: a single-center study. Ann Pediatr Endocrinol Metab 2022;27:52–9.




12. Kang HM, Jeong DC, Suh BK, Ahn MB. The impact of the coronavirus disease-2019 pandemic on childhood obesity and vitamin D status. J Korean Med Sci 2021;36:e21.




13. Leitner DR, Fruhbeck G, Yumuk V, Schindler K, Micic D, Woodward E, et al. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies -EASO can lead the way. Obes Facts 2017;10:483–92.




15. Weihrauch-Bluher S, Wiegand S. Risk factors and implications of childhood obesity. Curr Obes R ep 2018;7:254–9.


16. Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes 2021;12:344–65.



17. Drake AJ, Smith A, Betts PR, Crowne EC, Shield JP. Type 2 diabetes in obese white children. Arch Dis Child 2002;86:207–8.



18. Schober E, Holl RW, Grabert M, Thon A, Rami B, Kapellen T, et al. Diabetes mellitus type 2 in childhood and adolescence in Germany and parts of Austria. Eur J Pediatr 2005;164:705–7.



19. Ha J, Oh YR, Kang E, Nam HK, Rhie YJ, Lee KH. Single Point Insulin Sensitivity Estimator for predicting type 2 diabetes mellitus in obese adolescents. Ann Pediatr Endocrinol Metab 2022;27:201–6.




20. Hong YH, Chung IH, Han K, Chung S. Prevalence of type 2 diabetes mellitus among Korean children, adolescents, and adults younger than 30 years: changes from 2002 to 2016. Diabetes Metab J 2022;46:297–306.



21. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes 2013;4:270–81.



22. La Sala L, Pontiroli AE. New fast acting glucagon for recovery from hypoglycemia, a life-threatening situation: nasal powder and injected stable solutions. Int J Mol Sci 2021;22:10643.



23. Marks BE, Khilnani A, Meyers A, Flokas ME, Gai J, Monaghan M, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr 2021;94:275–84.



24. Lee MS, Lee R, Ko CW, Moon JE. Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic: a retrospective cross-sectional study. J Yeungnam Med Sci 2022;39:46–52.



25. Magge SN, Wolf RM, Pyle L, Brown EA, Benavides VC, Bianco ME, et al. The coronavirus disease 2019 pandemic is associated with a substantial rise in frequency and severity of presentation of youth-onset type 2 diabetes. J Pediatr 2022;251:51–9.e2.



26. Shin CH. Impact of COVID-19 pandemic on pediatric diabetes mellitus. J Korean Med Sci 2022;37:e186.




27. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr 2018;61:135–49.




28. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140:e20171904.

29. Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol 1988;62:345–6.


30. Lim JS, Kim EY, Kim JH, Yoo JH, Yi KH, Chae HW, et al. 2017 Clinical practice guidelines for dyslipidemia of Korean children and adolescents. Ann Pediatr Endocrinol Metab 2020;25:199–207.




31. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128 Suppl 5(Suppl 5):S213–56.



32. Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012;54:700–13.


33. Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol 2021;6:864–73.


34. Nur S. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int 2021;2021:1497449.


35. Gavin B, Lyne J, McNicholas F. The global impact on mental health almost 2 years into the COVID-19 pandemic. Ir J Psychol Med 2021;38:243–6.


36. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep 2014;14:508.




37. Biamonte E, Pegoraro F, Carrone F, Facchi I, Favacchio G, Lania AG, et al. Weight change and glycemic control in type 2 diabetes patients during COVID-19 pandemic: the lockdown effect. Endocrine 2021;72:604–10.




38. Onmez A, Gamsizkan Z, Ozdemir S, Kesikbas E, Gokosmanoglu F, Torun S, et al. The effect of COVID-19 lockdown on glycemic control in patients with type 2 diabetes mellitus in Turkey. Diabetes Metab Syndr 2020;14:1963–6.



39. Brooks CG, Spencer JR, Sprafka JM, Roehl KA, Ma J, Londhe AA, et al. Pediatric BMI changes during COVID-19 pandemic: an electronic health record-based retrospective cohort study. EClinicalMedicine 2021;38:101026.



40. Ge W, Hu J, Xiao Y, Liang F, Yi L, Zhu R, et al. COVID-19‒related childhood BMI increases in China: a health surveillance‒based ambispective cohort analysis. Am J Prev Med 2022;63:647–55.



41. Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T, et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity (Silver Spring) 2020;28:1382–5.




- TOOLS
- Related articles in APEM