 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 20(1); 2015 > Article |
|
Abstract
Purpose
Methods
Results
Conclusion
References
Fig. 1
Schematic representation of diabetic patients with persistent, regression, and progression groups to microalbuminuria. T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
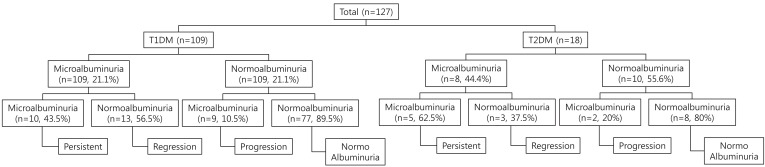
Fig. 2
Comparison of spot urine microalbumin/creatinine ratio between baseline and follow-up assessment. The median is depicted by the horizontal line, the interquartile range by the box limits, ranges by the whiskers, and outliers by the circles.
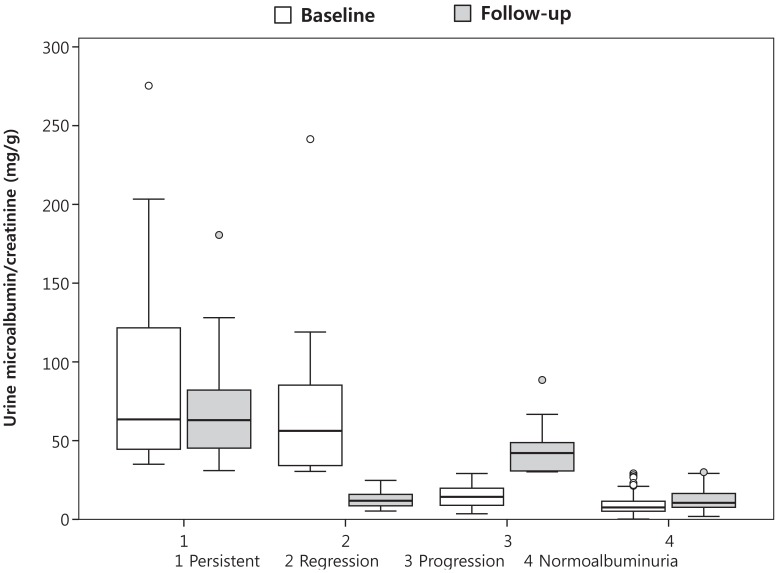
Table 1.
Anthropometric and laboratory characteristics of patients with type 1 and type 2 diabetes
| Variable |
T1DM (n=109) |
T2DM (n=18) |
||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Anthropometric index | ||||
| Age (yr) | 17.5a)(15.1–20.5) | 18.9 (16.5–21.0) | 15.4a)(12.6–17.4) | 17.9 (16.8–18.4) |
| Sex, male/female | 39/70 | 4/14 | ||
| Diabetes duration (yr) | 9.5a)(6.5–13.5) | 10.1 (7.8–14.0) | 0.9a)(0.0–3.0) | 5.0 (3.5–5.6) |
| Height (cm) | 162.0a)(160.0–170.0) | 162.9 (160.0–172.0) | 158.5a)(153.5–160.5) | 160.2 (153.5–163.2) |
| Weight (kg) | 57.0a)(53.0–64.0) | 58.5 (54.0–65.0) | 61.0 (54.0–73.7) | 63.4 (54.0–75.5) |
| BMI (kg/m2) | 21.52a)(19.53–23.15) | 21.91 (20.03–23.41) | 24.74 (21.09–30.88) | 24.31 (21.09–29.02) |
| Height z-score | 0.12a)(–0.44 to 1.19) | 0.05 (–0.55 to 0.86) | 0.50a)(–0.15 to 0.71) | –0.10 (–1.45 to 0.13) |
| Weight z-score | 0.31 (–0.17 to 1.00) | 0.27 (–0.17 to 1.02) | 1.02a)(–0.01 to 2.50) | 0.35 (–0.01 to 2.17) |
| BMI z-score | 0.31 (–0.43 to 0.78) | 0.30 (–0.43 to 0.83) | 1.29 (–0.02 to 2.49) | 0.99 (–0.01 to 2.27) |
| Biochemical profiles | ||||
| Serum creatinine (mg/dL) | 0.80 (0.70–0.90) | 0.75 (0.69–0.85) | 0.80 (0.60–0.90) | 0.65 (0.60–0.76) |
| eGFR (mL/min/1.73 m2) | 125.7 (110.0–134.6) | 127.9a)(118.8–136.1) | 128.5 (109.9–156.3) | 139.6 (129.0–152.1) |
| Urine microalbumin/creatinine (mg/g) | 9.4 (6.3–26.6) | 13.3 (8.6–24.8) | 25.3 (11.5–56.0) | 17.7 (6.2–42.0) |
| Cholesterol (mg/dL) | 175.0 (167.0–230.0) | 179.0 (163.0–206.0) | 194.0 (161.0–231.0) | 150.0 (139.0–222.0) |
| TG (mg/dL) | 79.0 (61.0–101.0) | 74.0 (56.0–93.0) | 105.0 (85.0–235.0) | 92.0 (64.0–262.0) |
| HDL-cholesterol (mg/dL) | 58.0 (50.0–68.0) | 60.0a)(52.0–68.0) | 49.5 (43.0–52.0) | 53.0 (45.0–59.0) |
| HbA1c (%) | 8.3 (7.3–9.6) | 8.4 (7.4–9.6) | 8.8 (6.9–12.2) | 7.8 (6.2–11.7) |
| C-peptide (ng/mL) | 0.02 (0.015–0.039) | 0.02 (0.015–0.018) | 2.97a)(2.471–3.733) | 1.96 (1.432–2.393) |
Values are presented as median (interquartile range) or number.
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; BMI, body mass index; eGFR, estimated glomerular filtration rate; TG, triglyceride; HDL-cholesterol, high density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; C-peptide, connecting peptide.
Table 2.
Characteristics of patients with type 1 diabetes according to microalbuminuria status (n=109)
| Characteristic |
Persistent (n=10) |
Regression (n=13) |
Progression (n=9) |
Normal (n=77) |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Anthropometric index | ||||||||
| Age (yr) | 17.8 (14.5-22.5) | 19.2 (17.0-23.0) | 17.0 (15.0-21.0) | 20.0 (17.5-21.5) | 19.0 (19.0-24.5) | 20.0 (19.5-25.0) | 17.5 (15.0-20.5) | 18.0 (15.5-21.0) |
| Sex, male/female | 2/8 | 4/9 | 3/6 | 4/9 | ||||
| Diabetes duration (yr) | 12.0 (7.5-16.0) | 12.8 (8.0-16.5) | 10.0 (5.0-11.1) | 10.5 (7.9-13.0) | 11.5 (5.5-15.0) | 12.0 (7.5-16.0) | 9.5 (6.5-13.5) | 10.0 (7.0-14.0) |
| Follow-up duration (yr) | 0.5 (0.5-1.0)a) | 1.0 (0.5-4.0) | 0.5 (0.5–1.0)b) | 1.0 (0.5-0.5) | ||||
| Height z-score | 0.10 (-0.15-0.63) | 0.11 (-0.15 to 0.53) | -0.15 (-0.41 to 0.28) | -0.22 (-0.55 to 0.26) | 0.01 (-0.15 to 0.25) | -0.01 (-0.15 to 0.25) | 0.24 (-0.55 to 1.02) | 0.15 (-0.55 to 0.86) |
| Weight z-score | 0.67 (0.32-1.13) | 0.68 (0.04-1.02) | 0.42 (-0.10-0.71) | 0.22 (-0.09 to 1.13) | 0.08 (-0.02 to 0.79) | 0.08 (-0.02 to 0.79) | 0.29 (-0.17 to 0.90) | 0.29 (-0.17 to 0.90) |
| BMI z-score | 0.55 (0.28-0.83) | 0.51 (0.12-0.83) | 0.39 (-0.12 to 0.76) | 0.34 (0.30-0.68) | -0.04 (-0.47 to 0.77) | -0.04 (-0.11 to 0.77) | 0.28 (-0.50 to 0.59) | 0.25 (-0.50 to 0.59) |
| Biochemical profiles | ||||||||
| sCr (mg/dL) | 0.70 (0.60-0.90) | 0.69 (0.65-0.74) | 0.80 (0.70-1.00) | 0.73 (0.69-0.99) | 0.78 (0.60-0.80) | 0.75 (0.74-0.81) | 0.80 (0.70-0.90) | 0.76 (0.69-0.86) |
| eGFR (mL/min/1.73 m2) | 127.7 (110.0-165.0) | 129.5 (125.3-156.6) | 116.3 (99.4-127.3) | 128.3 (119.3-135.4) | 117.3 (116.9-148.5) | 118.8 (116.5-128.8) | 125.7 (110.0-133.6) | 127.5 (117.0-136.1) |
| Urine microalbumin/creatinine (mg/g) | 63.3 (48.9-171.8) | 58.0 (48.0-92.4) | 56.4 (34.1-74.0) | 9.6 (8.6-15.8) | 15.4 (11.4-22.0) | 42.1 (30.8-50.2) | 7.4 (5.0-10.1) | 11.3 (7.8-16.5) |
| Cholesterol (mg/dL) | NA | NA | 175.0 (160.0-214.0) | 168.0 (163.0-206.0) | NA | NA | 181.0 (175.0-187.0) | 177.5 (160.0-195.0) |
| TG (mg/dL) | 70.0 (64.0-135.0) | 75.0 (68.0-119.0) | 79.5 (67.0-109.0) | 77.5 (73-94) | 92.0 (81-94) | 71.0 (61-89) | 76.0 (56-99) | 67.0 (53-91) |
| HDL-cholesterol (mg/dL) | 58.0 (56.0-64.0) | 55.0 (53.0-66.0) | 58.0 (53.0-67.0) | 61.5 (55.0-68.0) | 56.0 (45.0-68.0) | 60.0 (47.0-75.0) | 58.0 (49.0-67.0) | 60.0 (52.0-68.0) |
| HbA1c (%) | 9.8 (8.7-13.8)c) | 9.7 (8.9-10.1)d) | 8.0 (7.0-8.9)e) | 8.0 (7.1-9.4)f) | 9.6 (8.5-10.2) | 9.5 (7.8-10.1) | 8.2 (7.1-9.1) | 8.1 (7.2-9.0) |
| C-peptide (ng/mL) | 0.02 (0.015-0.015) | 0.02 (0.015-0.018) | 0.27 (0.015-0.571)g) | 0.02 (0.020-0.050) | 0.03 (0.015-0.100)h) | 0.04 (0.020-0.092) | 0.02 (0.015-0.020) | 0.02 (0.015-0.022) |
Values are presented as median (interquartile range or range) or number.
T1DM, type 1 diabetes mellitus; BMI, body mass index; sCr, serum creatinine; eGFR, estimated glomerular filtration rate; TG, triglyceride; HDL-cholesterol, high density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; C-peptide, connecting peptide; NA, not available.
Table 3.
Characteristics of patients with type 2 diabetes according to microalbuminuria status (n=18)
| Characteristic |
Persistent (n=5) |
Regression (n=3) |
Progression (n=2) |
Normoalbuminuria (n=8) |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Anthropometric index | ||||||||
| Age (yr) | 14.9 (13.3-16.8) | 17.9 (17.8-18.1) | 14.1 (11.9-26.0) | 18.2 (13.8-27.0) | 18.9 (15.8-22.0) | 19.7 (16.8-22.5) | 14.4 (12.3-17.4) | 17.0 (15.8-18.4) |
| Sex, male/female | 0/3 | 0/5 | 0/2 | 4/4 | ||||
| Diabetes duration (yr) | 2.6 (0.2-3.0) | 5.6 (5.3-6.6) | 0.0 (0.0-13.0) | 4.1 (1.9-14.0) | 2.3 (0.5-4.0) | 3.0 (1.0-5.0) | 0.7 (0.0-3.0) | 4.6 (3.2-5.1) |
| Follow-up duration (yr) | 4.4 (3.0-5.1) | 1.9 (1.0-4.1) | 0.8 (0.5-1.0) | 2.6 (0.5-5.0) | ||||
| Height z-score | 0.00 (-0.02 to 0.01)a) | -0.02 (-1.45 to -0.01) | -0.15 (-0.55 to 0.05) | -0.63 (-0.79 to -0.15) | -1.92 (-2.23 to -1.60) | -1.92 (-2.23 to -1.61) | 0.65 (0.46-0.98) | 0.12 (-0.70 to 0.47) |
| Weight z-score | 2.33 (1.18-2.50) | 1.56 (1.47-2.38) | -0.15 (-2.16 to -0.02) | -0.02 (-3.07-0.04) | 0.20 (0.13-0.27) | 0.20 (0.13-0.27) | 1.74 (0.31-2.68) | 0.63 (-0.01 to 2.29) |
| BMI z-score | 2.10 (1.28-2.85) | 2.27 (1.57-2.35) | -0.26 (-2.39 to -0.02) | -0.02 (-2.97 to 0.49) | 1.16 (1.00-1.31) | 1.13 (0.94-1.31) | 1.83 (-0.07 to 2.57) | 0.99 (-0.16 to 2.27) |
| Biochemical profiles | ||||||||
| sCr (mg/dL) | 0.80 (0.58-0.90) | 0.59 (0.49-0.64) | 0.80 (0.52-1.10) | 0.62 (0.55-0.76) | 0.63 (0.60-0.65) | 0.71 (0.66-0.76) | 0.85 (0.65-0.90) | 0.75 (0.61-0.81) |
| eGFR (mL/min/1.73 m2) | 110.3 (99.3-151.9) | 152.1 (131.8-180.4) | 115.8 (77.5-158.7) | 136.2 (110.0-157.5) | 132.9 (128.2-137.5) | 117.7 (108.6-126.8) | 131.3 (109.9-181.5) | 145.2 (131.1-148.6) |
| Urine microalbumin/creatinine (mg/g) | 63.48 (40.18-117.46) | 68.59 (42.04-72.26) | 56.00 (34.29-241.43) | 15.29 (13.23-20.02) | 6.21 (3.55-8.88) | 38.76 (30.20-47.32) | 13.16 (9.29-21.32) | 5.96 (3.83-9.13) |
| Cholesterol (mg/dL) | 218.0 (194-253) | 209.0 (147-338) | 196.0 (161-231) | 174.0 (160-188) | NA | NA | 162.0 (136-165) | 139.0 (136-141) |
| TG (mg/dL) | 295.0 (179-581)b) | 326.0 (320-572)c) | 87.0 (85-251) | 95.0 (60-149) | 181.0 (127-235) | 215.0 (168-262) | 68.5 (55-106) | 64.0 (60-82) |
| HDL-cholesterol (mg/dL) | 52.0 (51-52) | 54.0 (45-57) | 64.0 (41-73) | 59.0 (44-64) | 39.5 (35-44) | 43.5 (41-46) | 47.5 (43-50) | 53.0 (45-66) |
| HbA1c (%) | 11.2 (7.0-12.2) | 11.1 (8.2-12.3) | 13.3 (7.6-15.0) | 6.3 (6.1-12.1) | 6.7 (6.1-7.3) | 7.8 (6.4-9.2) | 8.3 (5.6-11.1) | 6.6 (5.4-11.7) |
| C-peptide (ng/mL) | 3.73 (2.710-3.750) | 2.25 (1.960-3.100) | 2.08 (1.010-2.230) | 2.43 (1.420-3.440) | 3.41 (2.470-4.340) | 1.83 (1.270-2.390) | 3.00 (2.970-3.200) | 1.63 (1.180-2.130) |
Values are presented as median (interquartile range or range) or number.
T2DM, type 2 diabetes mellitus; BMI, body mass index; sCr, serum creatinine; eGFR, estimated glomerular filtration rate; TG, triglyceride; HDL-cholesterol, high density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; C-peptide, connecting peptide; NA, not available.
- TOOLS
-
METRICS

-
- 8 Crossref
- Scopus
- 9,765 View
- 138 Download





